Anti Hcv
The presence of antiHCV in a blood sample indicates past or present infection with the virus Medical Dictionary, © 09 Farlex and Partners Want to thank TFD for its existence?.

Anti hcv. Interpretive Information A nonreactive test result does not exclude the possibility of exposure to or infection with hepatitis C virus Nonreactive test results in individuals with prior exposure to HCV may be due to antibody levels being below the detection limit of this assay or to lack of antibody reactivity to the recombinant antigens used in this assay. METHODS AntiHCV was assayed in serum by secondgeneration enzymelinked immunosorbent assay (ELISA), considering a serum antiHCV (), when the optical density ratio was equal to or greater than three times the cutoff value, in duplicate determinations, whereas antiH, antiHBs, HBsAg, antiHBe, and HBeAg were also evaluated by ELISA, as. Package insert Abbott Laboratories Architect AntiHCV product insert, Nov 15 Test Classification and CPT Coding Hepatitis C antibody Hepatitis C antibody confirmation (if appropriate) Additional Information For BJH Laboratory Use Only.
The antibody is not protective When cases are unclear or when suspicion for hepatitis C is high, HCV RNA is measured AntiHCV usually appears within 2 weeks of acute infection but is sometimes delayed;. The Alinity s AntiHCV assay is a chemiluminescent microparticle immunoassay (CMIA) used for the qualitative detection of antibodies to hepatitis C virus (HCV) in human serum and plasma specimens. Hepatitis C Virus Encoded Antigen (AntiHCV Assay) ABBOTT HCV EIA ;.
Chiron RIBA HCV 30 Strip Immunoblot Assay;. AntiHCV antibodies This blood test is the first and sometimes only one you may get Also called the ELISA screen, it checks for antibodies that your body releases to fight the virus These. In hepatitis C, serum antiHCV represents chronic, past, or acute infection;.
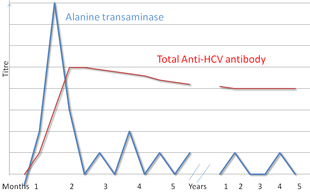
AntiHCV test results remain negative for several months after acute HCV infection Once antiHCV appears, it usually remains present for the life of the patient—even in the 15% of cases in whic. Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content. Hepatitis C antibodies are made by white blood cells and attack only the hepatitis C virus They bind to the virus and set it up for attack by other parts of the immune system The hepatitis C.
The last thing you want when being tested for Hepatitis C (HCV) is a falsepositive result HCV is a viral infection that affects the liver Unfortunately, false positives do occur. Hepatitis C antibody (HCV Ab, antiHCV) Hepatitis C for Patients This is the first test for determining whether you have been infected with hepatitis C The results will come back as either positive or negative. A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges No vaccine is currently available, but several vaccines are currently under development Most vaccines work through inducing an antibody response that targets the outer.
Autoimmune hepatitis (AIH) means your immune system attacks your liver cells Learn about the types, causes, risk factors, symptoms, diagnosis, treatment, and complications of autoimmune hepatitis. Hepatitis C virus (HCV) is a spherical, enveloped, positivestrand RNA virus Seven distinct HCV genotypes and 67 subtypes have been identified, the distribution of which vary geographically worldwide Approximately 70%–75% of people who seroconvert to antiHCV, indicative of acute infection, will progress to chronic infection and. Nucleic Acid Testing UltraQual HCV RT PCR Assay;.
The reflex algorithm allows 3 important HCV tests to be completed, if necessary, with a single blood draw Each of the 3 tests provides information that is important when a patient enters care1 • HCV antibody testing is the initial screening test. She has hepatitis c Antihcv is a test for antibodies to the hepatitis c virus A positive test means that a person has been exposed to hepatitis c A positive test means that a person has been exposed to hepatitis c. Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver;.
The VITROS AntiHCV test is performed using the VITROS AntiHCV Reagent Pack and VITROS Immunodiagnostic Products AntiHCV Calibrator on the VITROS ECi/ECiQ Immunodiagnostic Systems, the VITROS 3600 Immunodiagnostic System and the VITROS 5600 Integrated System An immunometric technique is used This involves a twostage reaction. The antiHCV test only provides information about past exposure to HCV A negative antiHCV result indicates that a patient has never been exposed to the virus, and therefore the antiHCV test is only used to rule out HCV infection. Introduction Laboratory tests for hepatitis C are divided into four general categories Screening Screening for hepatitis C virus (HCV) is done with a serologic test for the HCV antibody (Ab) Confirmatory Diagnosis of chronic hepatitis C requires the presence of HCV RNA, commonly called hepatitis C viral load Genotype Once it is determined that HCV RNA is present, the specific genotype.
تحليل HCVAB التهاب الكبد الوبائي C من الأمراض التي تُصيب الكبد، وتشيع الإصابة به في الدول النامية، ويُستخدم للكشف عنه فحص طبي يُسمى بفحص الأجسام المضادة لفيروس الكبد الوبائي C (بالإنجليزية Hepatitis C Virus Antibody) واختصاراً HCVAB. Compare AntiHCV Antibody Products from leading suppliers on Biocompare View specifications, prices, citations, reviews, and more. The Elecsys Anti‑HCV II assay is a thirdgeneration test6,7The Elecsys Anti‑HCV II assay uses peptides and recombinant antigens representing core, NS3 and NS4 proteins for the determination of.
The HCV antibody test, sometimes called the antiHCV test, looks for antibodies to the hepatitis C virus in blood Antibodies are chemicals released into the bloodstream when someone gets infected Test results can take anywhere from a few days to a few weeks to come back. A person whose antiHCV test is reactive should be tested for HCV RNA to distinguish active from cleared infection The recommended initial test is enzyme immunoassay (EIA) for HCV antibody The first enzyme immunoassay (EIA), which detected antibody to a single viral antigen, was introduced in 19. This screening test checks for antibodies against HCV (antiHCV) If the antibody test is positive, a PCR test is used to confirm HCV infection Further genetic testing is done to check for the type of HCV (genotype).
1,4 None of these antiHCV antibody tests can differentiate whether the infection is new (acute), chronic, or no longer present Enzyme Immunoassay (EIA) The thirdgeneration HCV EIA detects antibodies that bind to recombinant antigens derived from four HCV regions core, nonstructural 3, nonstructural 4, and nonstructural 5. The goal of treatment is to have no hepatitis C virus detected in your body at least 12 weeks after you complete treatment Researchers have recently made significant advances in treatment for hepatitis C using new, "directacting" antiviral medications, sometimes in combination with existing ones As a result, people experience better outcomes. ARCHITECT AntiHCV final reaction, bound acridinylated conjugates are If the chemilumninescent signal of the sample is greater than or equal to used to generate a cherifluminescentl signal, the cutoff signal, the sample is considered reactive for antiHCV HCV s a 3loodorneviru.
Elecsys™ AntiHCV II Roche Diagnostics Indianapolis, IN, USA ECLIA e (automated) OraQuick™ HCV Rapid Antibody Test OraSure Technologies, Inc Bethlehem, PA, USA Immunochromatographic (manual) Ortho HCV Version 30 ELISA Test System OrthoClinical Diagnostics, Inc Raritan, NJ, USA EIA a (manual) Vitros AntiHCV. Besides the great importance of the issue in terms of public health, there is a lack of studies evaluating the performance of several of the currently used point of care tests (POCTs) for the detection of antiHCVTo investigate the performance of two POCTs for antiHCV detection and to assess the impact of the reading time on diagnostic performanceA total of 307 subjects were divided into. AntiHCV ELISA is an enzymelinked immunosorbent assay (ELISA) for qualitative detection of antibodies to hepatitis C virus in human serum or plasma It is intended for screening blood donors and diagnosing patients related to infection with hepatitis C virus.
Article Title CoInfection of the Hepatitis C Virus With Other BloodBorne and Hepatotropic Viruses Among Hemophilia Patients in Poland Article Snippet Serological Tests The serological testing included antiHCV (Monolisa AntiHCV Plus version 2, BioRad), HIV Ag/Ab (Genscreen Ultra HIV AgAb, BioRad), HBsAg (Murex HBsAg version 3, Murex Biotech Limited), antiH (ETIABCorek Plus. Anti HCV Anti HCV Imunologie;. Chiron RIBA HCV 30 Strip Immunoblot Assay;.
Elecsys ® Anti‑HCV II is an immunoassay for the in vitro qualitative detection of antibodies to HCV in human serum and plasma 15 Hepatitis C Hepatitis C is an inflammatory liver disease caused by infection with the hepatitis C virus (HCV), which can cause both acute and chronic hepatitis1. Hepatitis C Virus Encoded Antigen (AntiHCV Assay) ABBOTT HCV EIA ;. It is a type of viral hepatitis During the initial infection people often have mild or no symptoms Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs The virus persists in the liver in about 75% to 85% of those initially infected.
1,4 None of these antiHCV antibody tests can differentiate whether the infection is new (acute), chronic, or no longer present Enzyme Immunoassay (EIA) The thirdgeneration HCV EIA detects antibodies that bind to recombinant antigens derived from four HCV regions core, nonstructural 3, nonstructural 4, and nonstructural 5. Test specific de depistare in sange a anticorpilor antivirus hepatita C Anticorpii nu sunt suficienti pentru a oferi imunitate organismului si testul nu poate face distinctia intre infectia acuta si cea cronica Daca testul este pozitiv se recomanda repetarea sa pentru eliminarea erorilor de laborator. Hepatitis C is a virus that damages the liver If left untreated, it can lead to liver disease and other serious longterm health problems Many people do not realize that they have hepatitis C.
However, antiHCV screening is of these variables performed in 1372 motherinfant pairs recommended in some circumstances, that should be demonstrated that only intravenous drug abuse was an accurately investigated in the clinical history of mothers, independent risk factor for HCV transmissionsuch as A great deal of research has been devoted. Nucleic Acid Testing UltraQual HCV RT PCR Assay;. Why reflex from a positive HCV antibody test result to quantitative HCV RNA and (if HCV RNA >300 IU/mL) to HCV genotype?.
Elecsys ® Anti‑HCV II is an immunoassay for the in vitro qualitative detection of antibodies to HCV in human serum and plasma15. The ARCHITECT AntiHCV assay is a twostep immunoassay, using chemiluminescent microparticle immunoassay (CMIA) technology, for the qualitative detection of antiHCV in human serum and plasma In the first step, sample, recombinant HCV antigen coated paramagnetic microparticles and Assay Diluent are combined. AntiHCV test results remain negative for several months after acute HCV infection Once antiHCV appears, it usually remains present for the life of the patient—even in the 15% of cases in whic.
Methods AntiHCV was assayed in serum by secondgeneration enzymelinked immunosorbent assay (ELISA), considering a serum antiHCV (), when the optical density ratio was equal to or greater than three times the cutoff value, in duplicate determinations, whereas antiH, antiHBs, HBsAg, antiHBe, and HBeAg were also evaluated by ELISA, as. Rata de transmisie verticală la gravidele cu antiHCV pozitiv a fost estimată la 2744%, această rată atingând valori de 5486% la gravidele care au coinfecţie VHC – HIV 1 Este bine de ştiut faptul că virusul nu se transmite prin folosirea în comun a veselei, piscinelor şi toaletelor 2. Drugs used to treat Hepatitis C The following list of medications are in some way related to, or used in the treatment of this condition Select drug class All drug classes miscellaneous antivirals (2) purine nucleosides (5) antineoplastic interferons (4) antiviral combinations (12) antiviral interferons (4) inhaled antiinfectives (5).
A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges No vaccine is currently available, but several vaccines are currently under development Most vaccines work through inducing an antibody response that targets the outer. The ritual of injecting antiHCV drugs, coupled with the potential side effect or occurrence of depression, may trigger relapses in substance use/abuse. However, HCV RNA is positive sooner.
With the emergence of new therapies that have transformed chronic hepatitis C virus (HCV) treatment, public health experts now believe that it is possible to eliminate the disease HCV elimination could have a tremendous impact on global health, averting the nearly 400,000 deaths from HCVrelated complications each year. Testing for antiHCV antibodies with a serological test identifies people who have been infected with the virus If the test is positive for antiHCV antibodies, a nucleic acid test for HCV ribonucleic acid (RNA) is needed to confirm chronic infection because about 30% of people infected with HCV spontaneously clear the infection by a strong. The hepatitis C antibody test is a blood test that looks for hepatitis C antibodies in the bloodstream A positive result usually means that you’ve been exposed to the hepatitis C virus.
The HCV RNA PCR test is used to determine whether the hepatitis C virus (HCV) exists in your bloodstream If the virus is present, the test can also measure the exact amount that’s in your blood. Hepatitis C is a virus that damages the liver If left untreated, it can lead to liver disease and other serious longterm health problems Many people do not realize that they have hepatitis C. That's changing Today, chronic HCV is usually curable with oral medications taken every day for two to six months Still, about half of people with HCV don't know they're infected, mainly because they have no symptoms, which can take decades to appear For that reason, the US Preventive Services Task Force recommends that all adults ages 18 to 79 years be screened for hepatitis C, even.
Chronic hepatitis C virus (HCV) infection has become a major public health burden worldwide Twentytwo sophocarpinic acid or matrine derivatives were synthesized and their antiHCV activities were evaluated in vitroThe structureactivity analysis revealed that (i) sophocarpinic acids with a Dseco 3ring structure scaffold were more favorable than matrines with a 4ring scaffold;. A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges No vaccine is currently available, but several vaccines are currently under development Most vaccines work through inducing an antibody response that targets the outer. The HCV RNA PCR test is used to determine whether the hepatitis C virus (HCV) exists in your bloodstream If the virus is present, the test can also measure the exact amount that’s in your blood.

Direct Anti Hcv Agents Sciencedirect
Http 158 232 12 119 Entity Hepatitis Publications Annex 6 1 Pdf
Plos One An Analysis On Hbsag Anti Hcv Anti Hiv And Vdrl Test Results In Blood Donors According To Gender Age Range And Years
Anti Hcv のギャラリー
Sensors Free Full Text Developments In The Hcv Screening Technologies Based On The Detection Of Antigens And Antibodies Html

Quantifying Antiviral Activity Optimizes Drug Combinations Against Hepatitis C Virus Infection Pnas
Clinical Guidelines For The Medical Management Of Hepatitis C
Q Tbn And9gcqinhuckak Giyr3hwbtf1f8u Tvoyzw6xz0vjbnydrku081sjz Usqp Cau

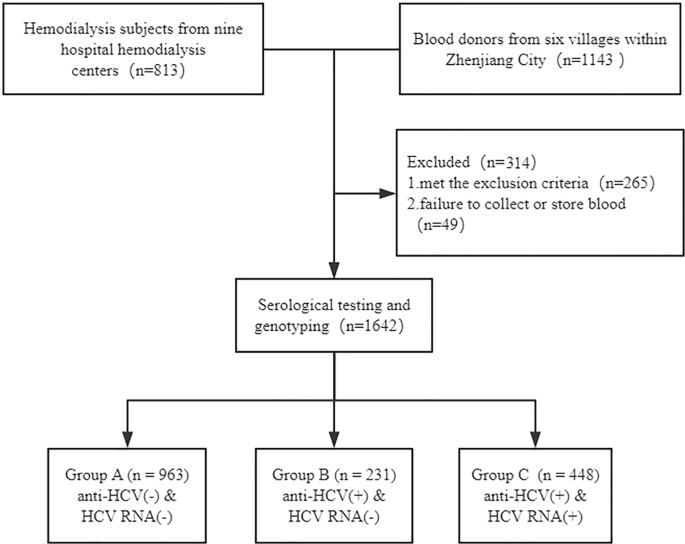
Characteristics Of Anti Hcv Positive In Two Groups Download Table
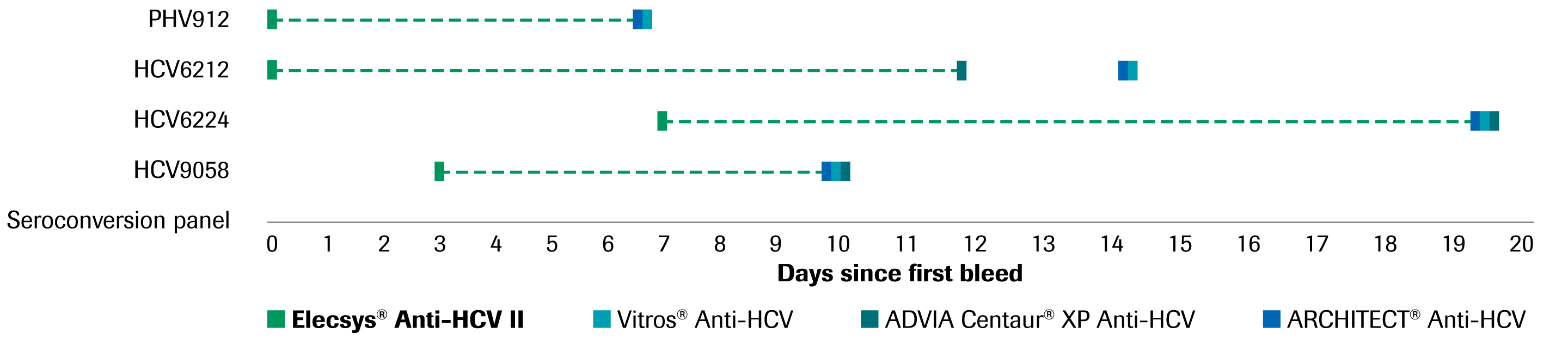
Elecsys Anti Hcv Ii

Bloedonderzoek Bij Verdenking Op Hepatitis C Hepatitis Info
Www Aphl Org Aboutaphl Publications Documents Id 19jan Hcv Test Result Interpretation Guide Pdf
Hepatitis C Virus Part 2 Anti Hcv Screening Labpedia Net

Anti Hcv Rochecanada Com English

Anti Hepatitis C Virus Anti Hcv Total Test Rs 300 Diagnosticcentres In

Hcv Core Antigen Is An Alternative Marker To Hcv Rna For Evaluating Active Hcv Infection Implications For Improved Diagnostic Option In An Era Of Affordable Daas Peerj
Hepatitis C Wikipedia
Testing And Clinical Management Of Health Care Personnel Potentially Exposed To Hepatitis C Virus Cdc Guidance United States Mmwr

Hepatitis C Virus Part 3 Anti Hcv Elisa Labpedia Net
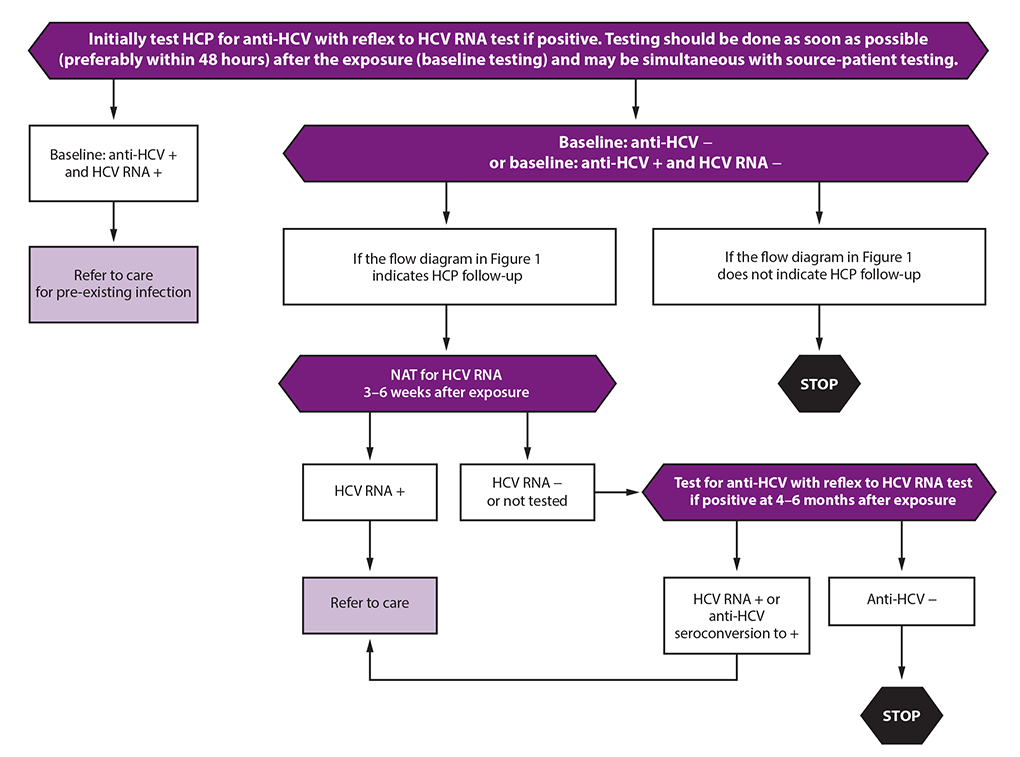
Testing And Clinical Management Of Health Care Personnel Potentially Exposed To Hepatitis C Virus Cdc Guidance United States Mmwr

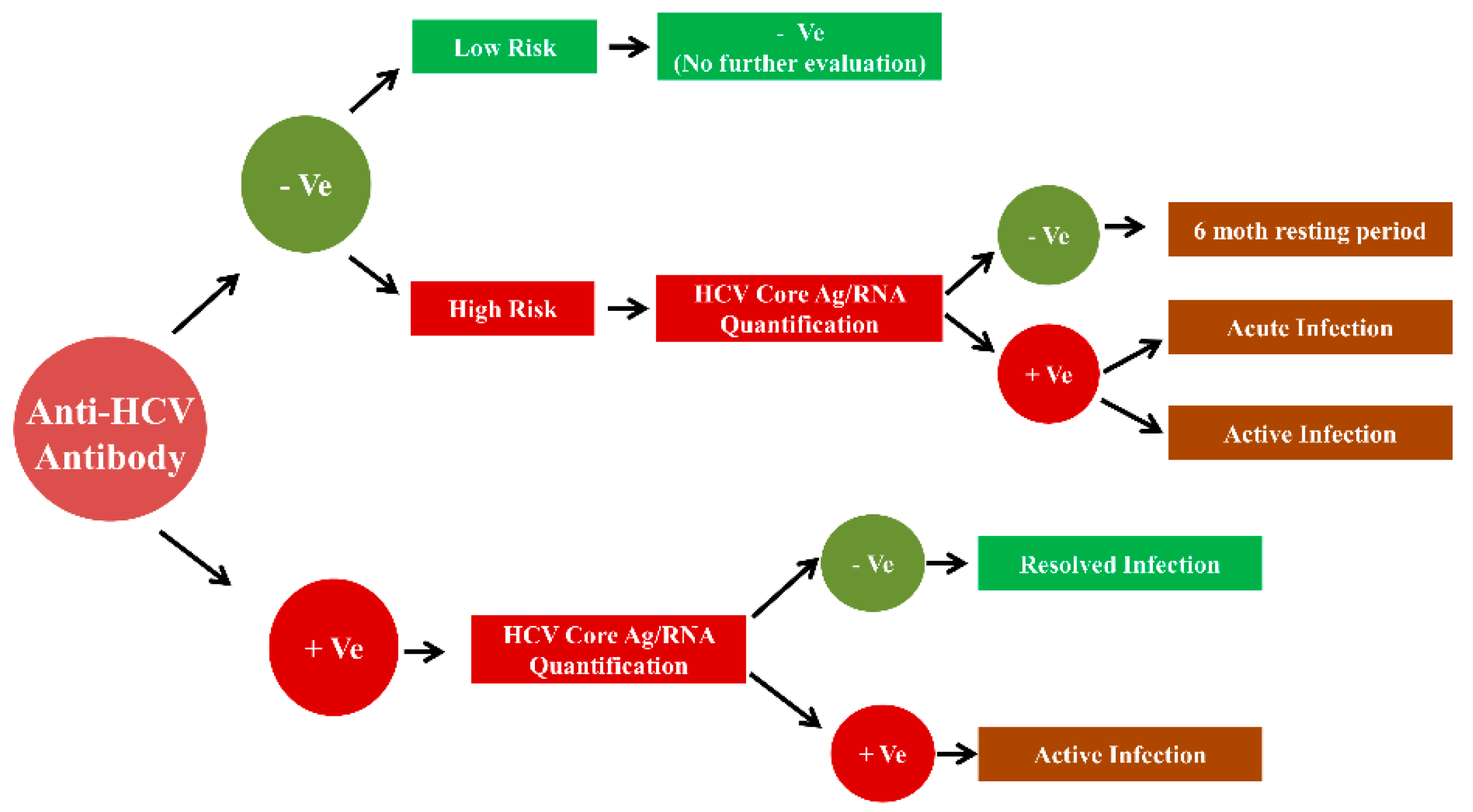
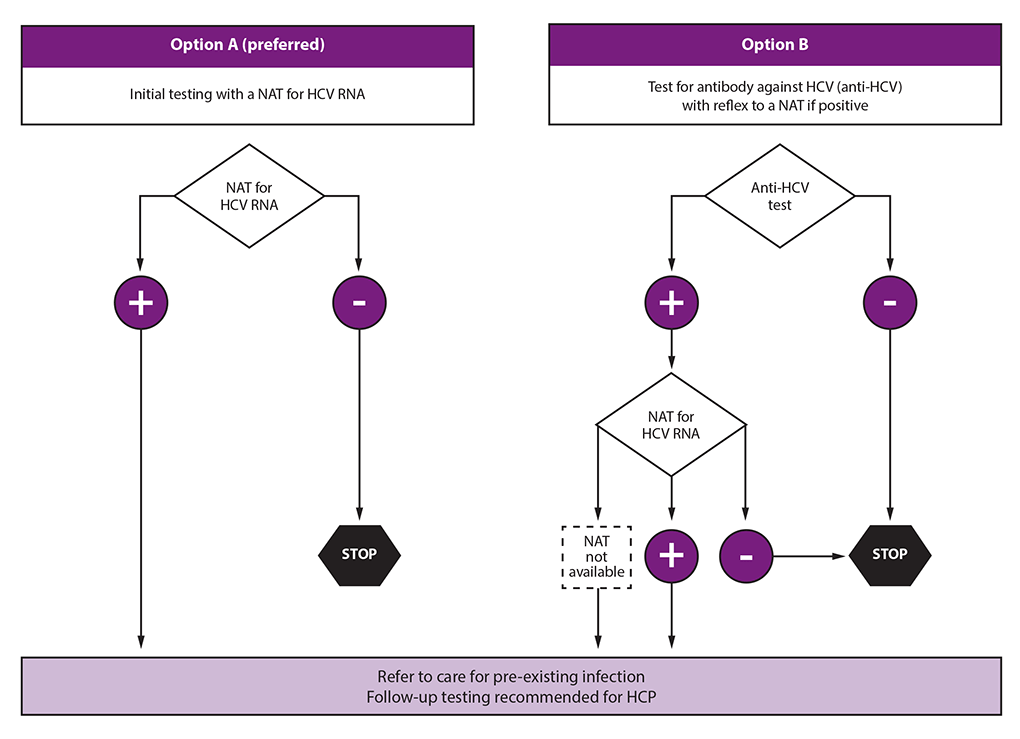
Fig 1b Two Assay Testing Strategy For Diagnosis Of Hcv Detection Of Anti Hcv Followed By Hcv Rna Core Ag Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf

Anti Hcv Cassette Anti Hcv Linear

The Korean Journal Of Internal Medicine

Features Of Anti Hcv Positive And Anti Hcv Negative Dialysis Patients Download Table

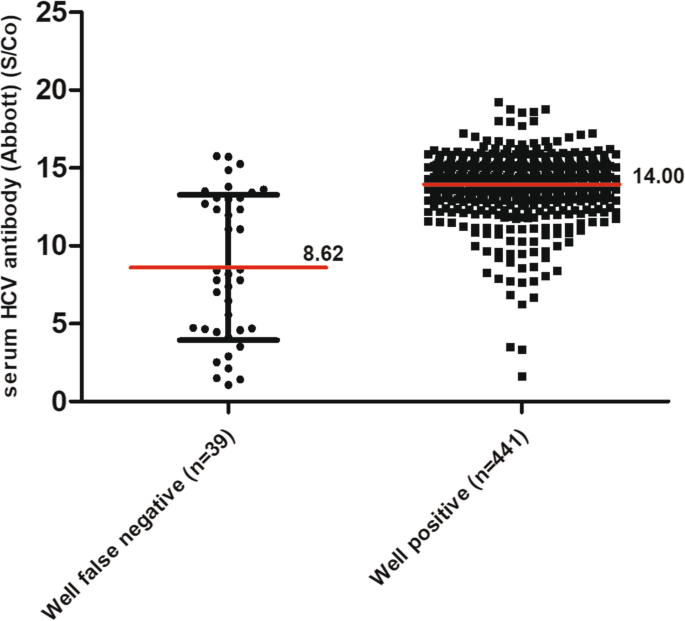
Role Of Signal To Cut Off Ratios Of Anti Hepatitis C Virus Antibody By Enzyme Immunoassays Along With Id Nat For Screening Of Whole Blood Donors In India Abstract Europe Pmc
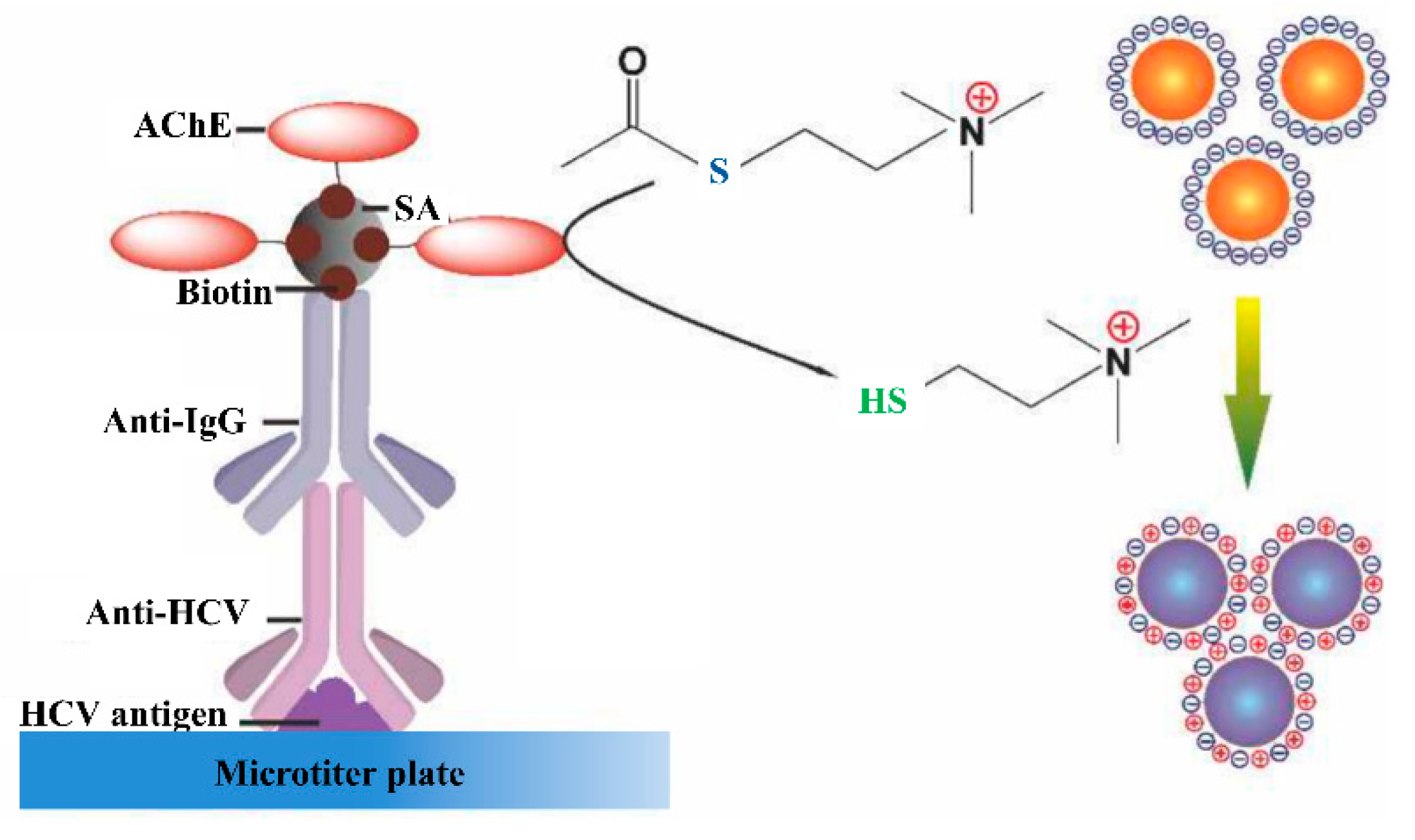
Sensors Free Full Text Developments In The Hcv Screening Technologies Based On The Detection Of Antigens And Antibodies Html

Anti Hepatitis C Virus Core Igm Antibodies Correlate With Hepatitis C Recurrence And Its Severity In Liver Transplant Patients Gut

Monolisa Anti Hcv Plus Version 3 Clinical Diagnostics Bio Rad
Www Banglajol Info Index Php Jafmc Article View
Schematic Diagram Of All 290 Anti Hcv Reactive Samples Anti Hcv Download Scientific Diagram

Characteristics Associated With Anti Hcv Serological Markers In Prisoners In The State Of Parana Brazil A Case Control Study

Top Pdf Anti Hcv Antibodies 1library

How To Screen For A Hcv Infection Early Youtube
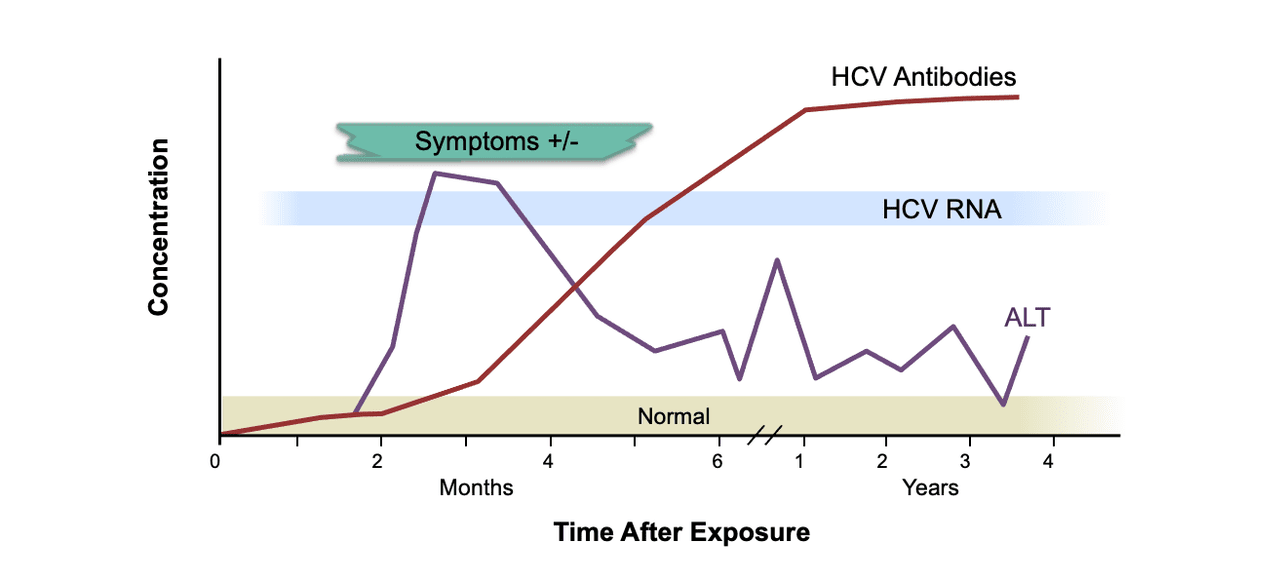
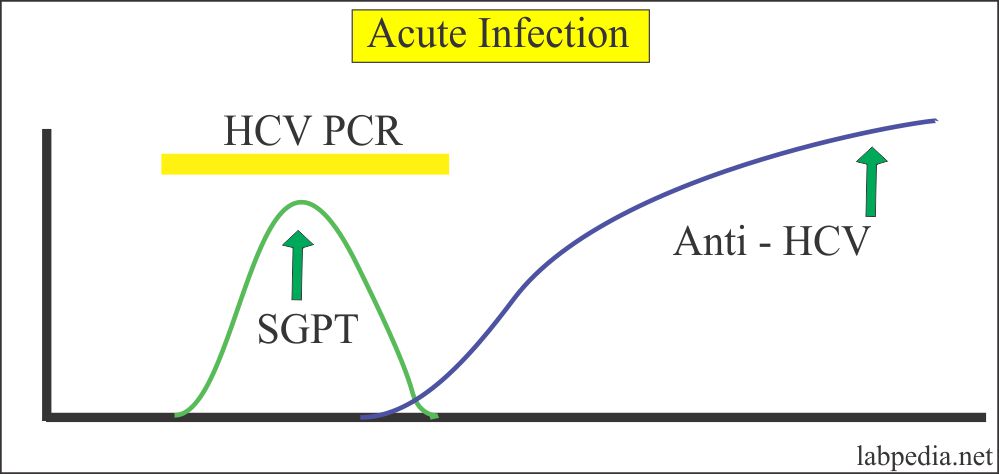
Diagnosis Of Acute Hcv Infection Core Concepts

Boditech Med Inc
Q Tbn And9gcqlksszwxrg4lhdivgijzbz65msd2iximnd Lueikbn Ylhgy1z Usqp Cau
Www Publichealthontario Ca Media Documents Hepc Iphis Data Entry Guide Pdf La En
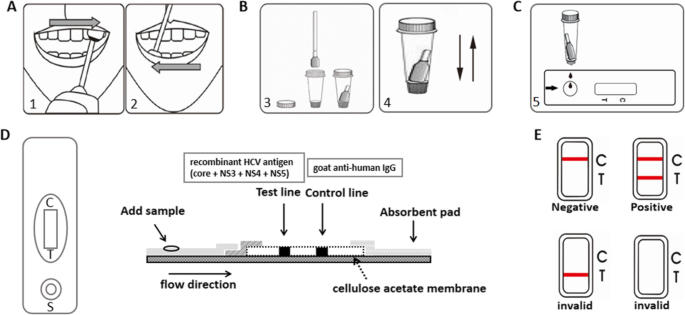
Evaluation Of A New Point Of Care Oral Anti Hcv Test For Screening Of Hepatitis C Virus Infection Virology Journal Full Text
Www Health Belgium Be Sites Default Files Uploads Fields Fpshealth Theme File De relevantie van nat Tests bij de beoordeling van donoren van menselijk lichaamsmateriaal 28november 11 29 28hgr 8684 29 Pdf
Comparison Stratagems Of Post Screening Management Of Anti Hcv Positive Community Residents Simple Notification Active Referral Or Accessible Medical Care

Diagnosis And Management Of Hepatitis C American Family Physician

Anti Hepatitis C Virus Core Igm Antibodies Correlate With Hepatitis C Recurrence And Its Severity In Liver Transplant Patients Gut

Screening And Linkage To Care For Hepatitis C Among Inpatients In Georgia S National Hospital Screening Program Sciencedirect
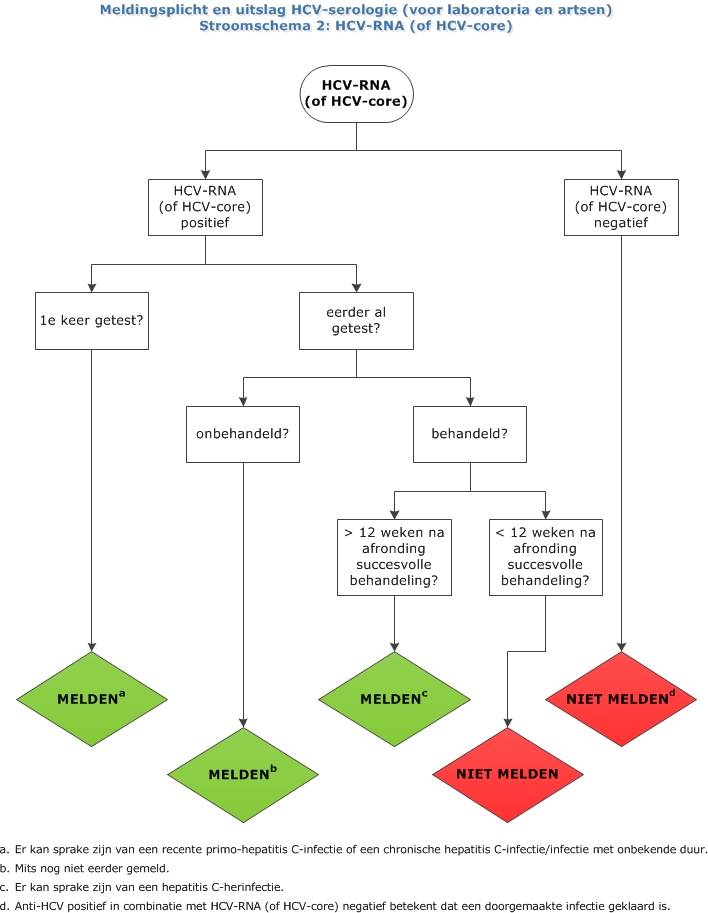
Hepatitis C Lci Richtlijnen
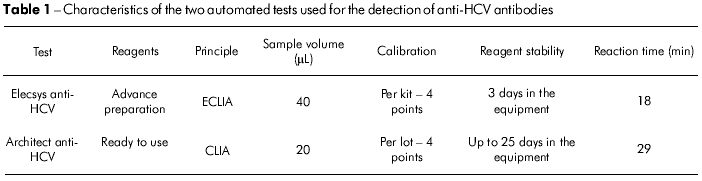
Comparacao De Dois Testes Automatizados Por Quimioluminescencia Para A Deteccao De Anticorpos Contra O Virus Da Hepatite C
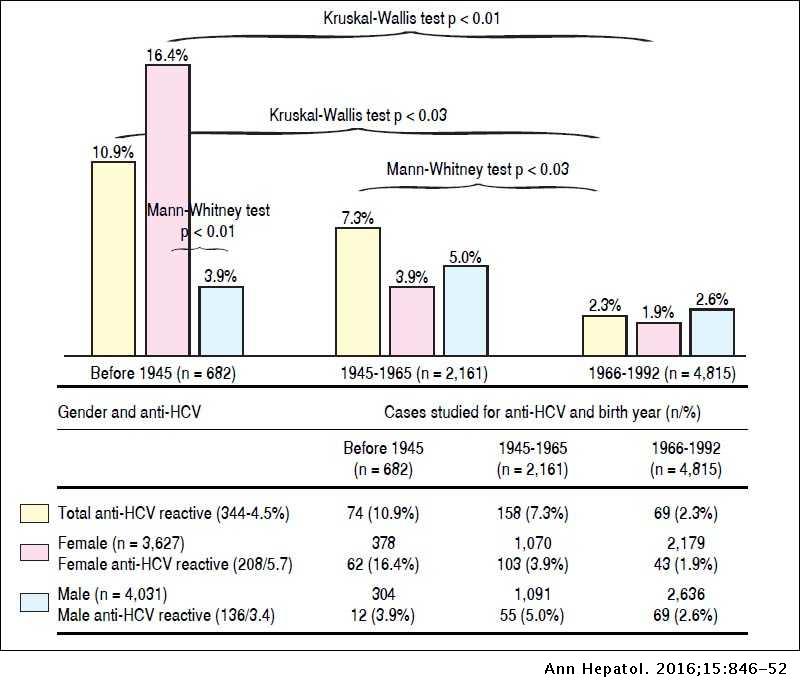
Frequency Of Hepatitis C Virus Infection In A Single Institution In Mexico With A Focus On Birth Cohort Population Annals Of Hepatology

Anti Hcv Drugs And Excretion Download Scientific Diagram
Http Www Ilexmedical Com Files Pdf Antihcv Arc Pdf
Www Accessdata Fda Gov Cdrh Docs Pdf3 Pb Pdf

Evaluation Of The Elecsys Anti Hcv Ii Assay For Routine Hepatitis C Virus Screening Of Different Asian Pacific Populations And Detection Of Early Infection Topic Of Research Paper In Biological Sciences Download

Anti Hcv Antibody Products Biocompare
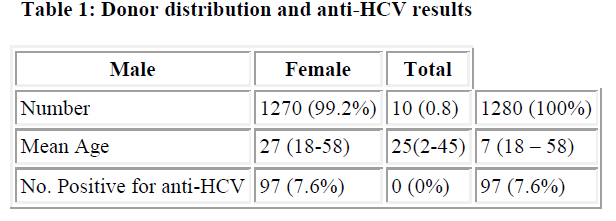
Seroprevalence Of Hepatitis C Virus Hcv Infection Among Blood Donors In A South Eastern State Of Nigeria
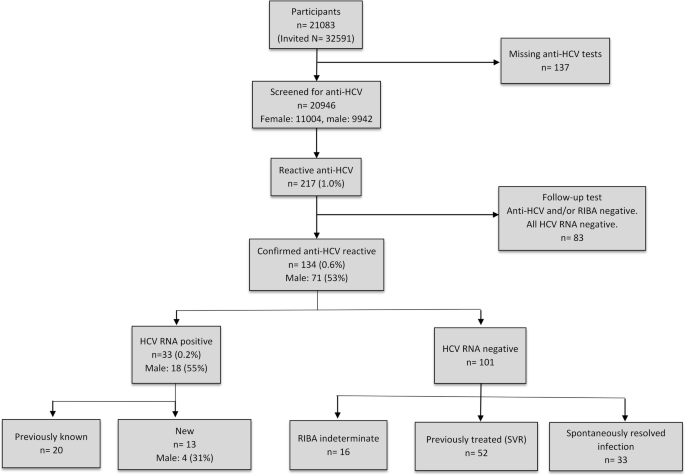
Screening For Hepatitis C In A General Adult Population In A Low Prevalence Area The Tromso Study Bmc Infectious Diseases Full Text
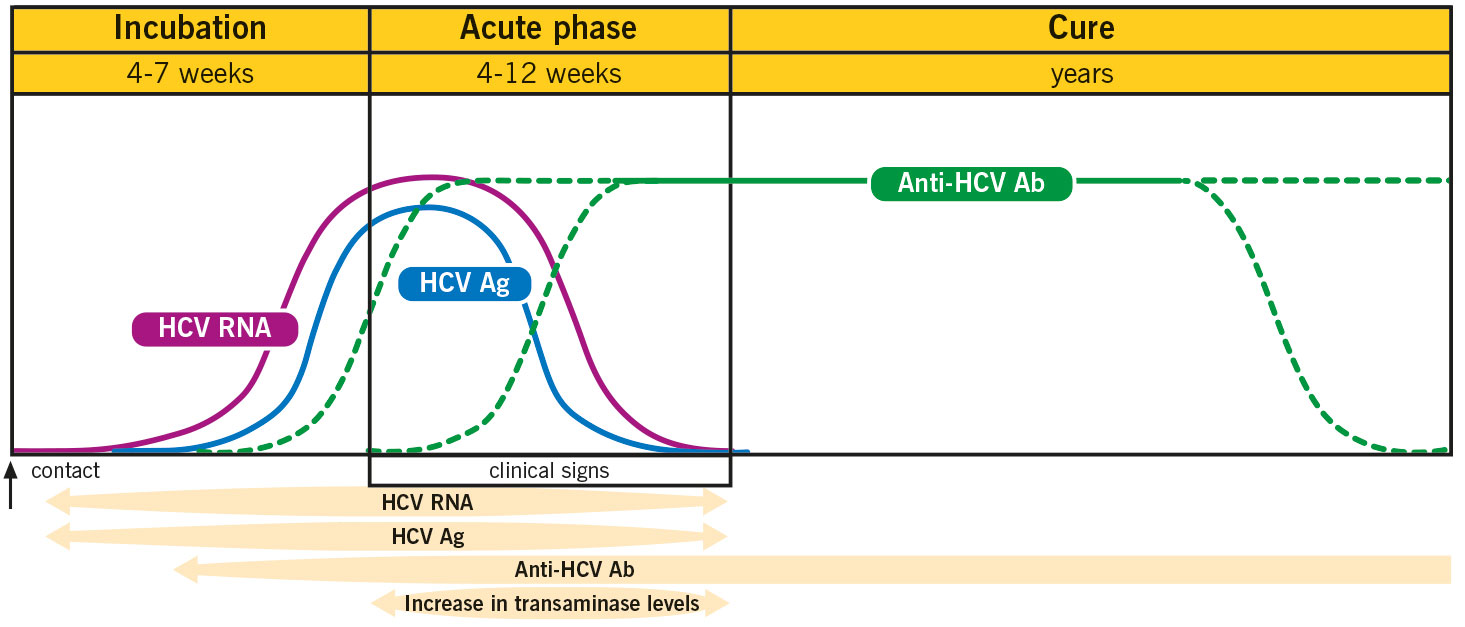
Hepatitis C Antibodies Information The Doctors Laboratory

Clinical Findings Of Hcv Chronic Infection In Undocumented Immigrants And Low Income Refugees In Three Areas Of Southern Italy Annals Of Hepatology
Genetic Mutations In Nf Kb Pathway Genes Were Associated With The Protection From Hepatitis C Virus Infection Among Chinese Han Population Scientific Reports
Www Internisten Nl Sites Internisten Nl Files Hcv stroomdiagram meldingsplicht en uitslag hcv serologie Pdf
Www Gov Mb Ca Health Publichealth Cdc Protocol Hepc Pdf

Effect Of Hla On Hepatitis C Virus Clearance And Persistence In Anti Hcv Positive End Stage Renal Disease Patients Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free
Afias Anti Hcv Avant Medical
Evaluation Of A New Point Of Care Oral Anti Hcv Test For Screening Of Hepatitis C Virus Infection Virology Journal Full Text
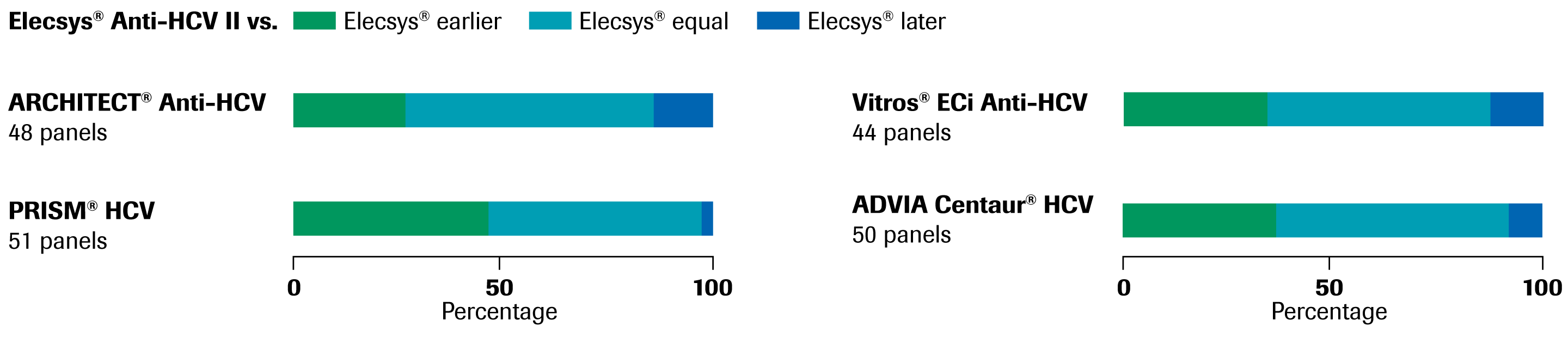
Elecsys Anti Hcv Ii
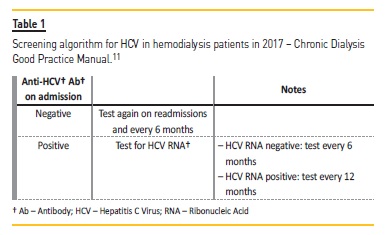
Hepatitis C Virus Core Antigen Test An Hcv Rna Screening Alternative In End Stage Renal Disease And Hemodialysis
Www Who Int Hepatitis Publications Annex 5 6 Pdf Ua 1
2
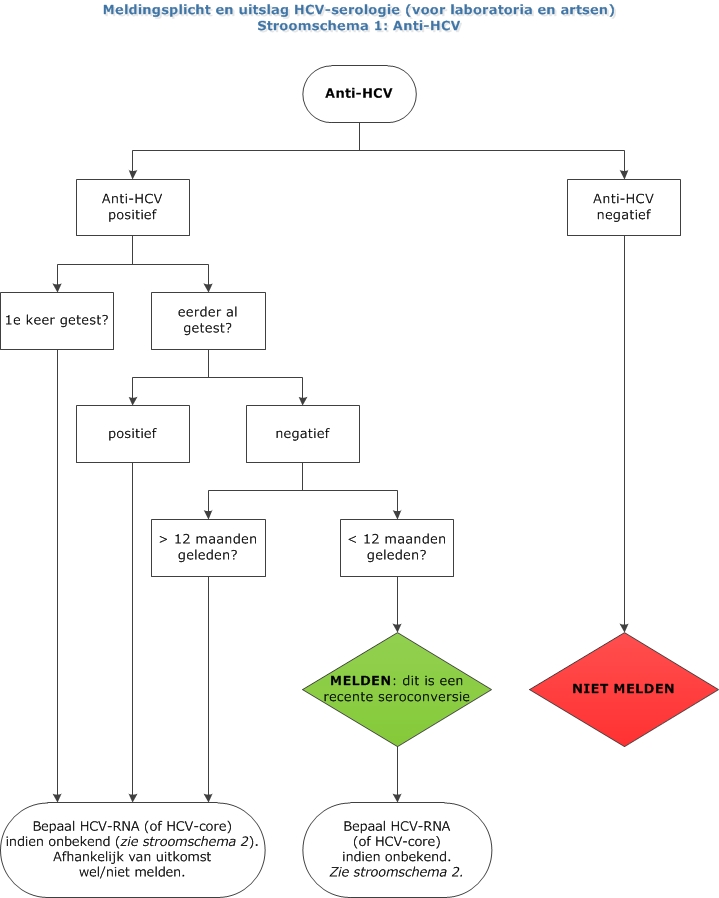
Hepatitis C Lci Richtlijnen

Quantitative Analysis Of Anti Hepatitis C Virus Antibody Secreting B Cells In Patients With Chronic Hepatitis C Umemura 06 Hepatology Wiley Online Library
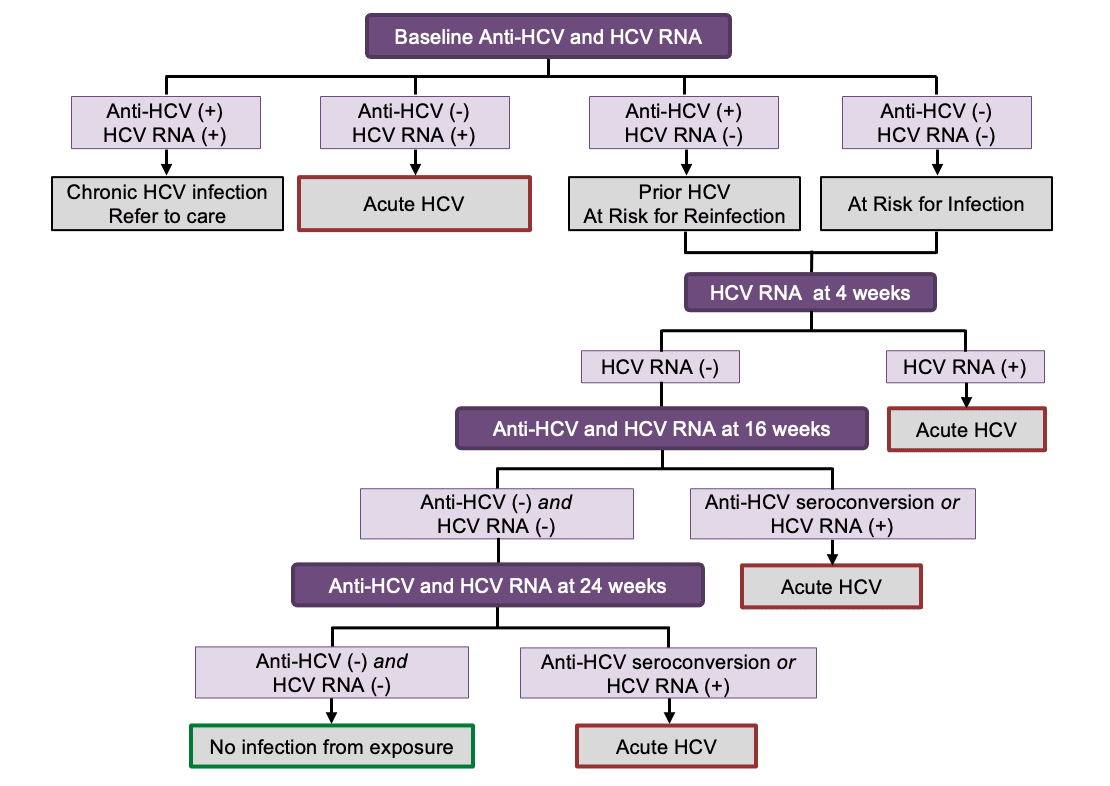
Diagnosis Of Acute Hcv Infection Core Concepts

The Korean Journal Of Internal Medicine
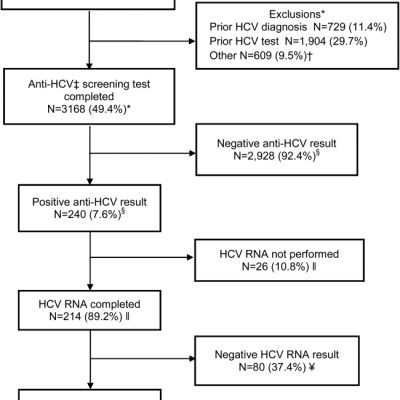
Baby Boomer Hcv Screening And Care Journal Of Hospital Medicine
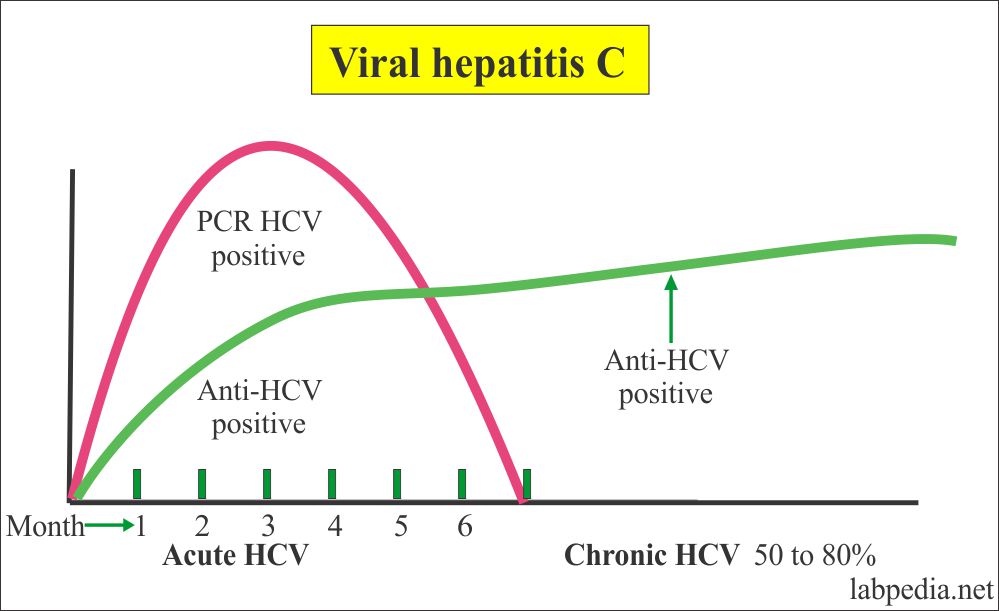
Hepatitis C Virus Part 1 Hepatitis C Virus Hcv Profile Labpedia Net
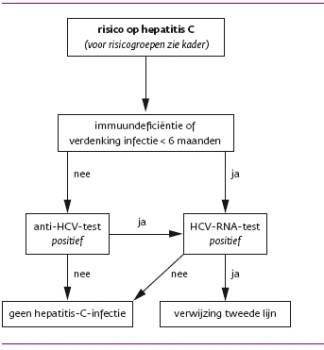
Hepatitis C Opsporing Verzocht Huisarts Wetenschap
Plos One High Prevalence Of Anti Hcv Antibodies In Two Metropolitan Emergency Departments In Germany A Prospective Screening Analysis Of 28 809 Patients

Comparison Of Anti Hcv Positive And Negative Patients Download Table
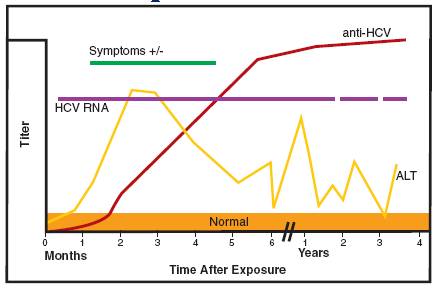
Hcv Rna Hepatitis C Virus Rna Nucleic Acid Amplification Test Newfoundland Labrador Public Health Laboratory
Q Tbn And9gcsf2 Pox2upnotm56whrd0khgrimaadlykabtxj Pdazc607ytg Usqp Cau

Prevalence Of Anti Hcv Antibodies Among Healthy Asymptomatic Indian B
2

The Evolution Of Occult Hepatitis C Virus After Immunosuppression In Advanced Ckd Patients Nefrologia English Edition

Evaluation Of The Novel Hiscl Chemiluminescence Enzyme Immunoassay For Laboratory Screening Of Hepatitis C Virus Clinical And Vaccine Immunology

The Kidney Transplant Recipient With Hepatitis C Infection Pre And Posttransplantation Treatment American Society Of Nephrology
Www Publichealthontario Ca En Erepository Lab Sd 034 Antihcv Results Next Steps Pdf

Shin Jin Medics Inc
Www Who Int Diagnostics Laboratory Evaluations Pq List Hcv Pqdx 0371 017 00 Pqpr Rapid Anti Hcv Test Pdf
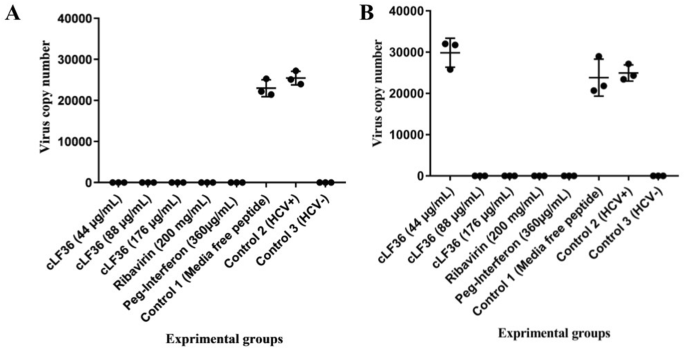
A Novel Chimeric Anti Hcv Peptide Derived From Camel Lactoferrin And Molecular Level Insight On Its Interaction With E2 Springerlink
Jidc Org Index Php Journal Article Download 2141

Intec Hcv Rapid Test Medtek
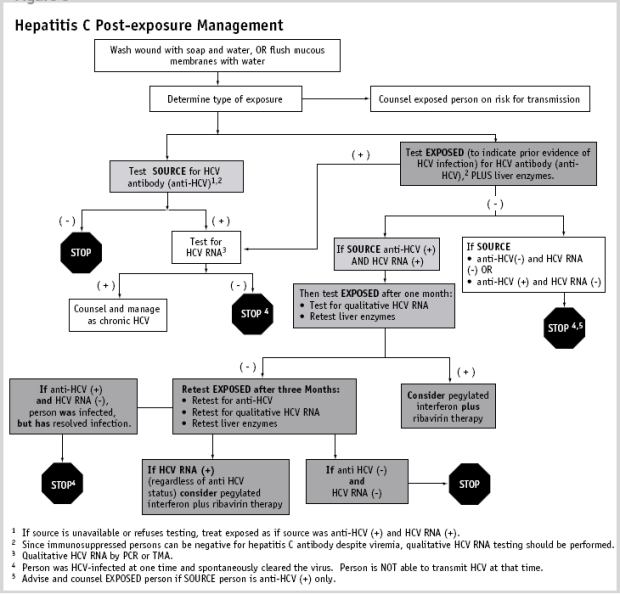
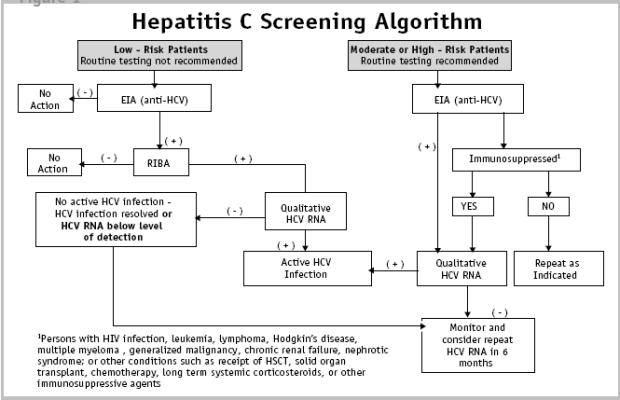
Clinical Guidelines For The Medical Management Of Hepatitis C

Hcv Elisa Kit Human Anti Hepatitis C Virus Hcv Antibody Elisa Kit Baj 1

Table Ii From Hepatitis C Virus Antibodies Anti Hcv In Haemodialyzed Vs Non Dialyzed Patients Semantic Scholar

Anti Hcv In Patients Undergoing Hemodialysis In Salvador Ba Brazil

Evaluation Of Assay Methods And False Positive Results In The Laboratory Diagnosis Of Hepatitis C Virus Infection Insight Medical Publishing

China Medical Diagnostic Anti Hcv Test Kits Hcv Rapid Test Kit Ce Approved Hepatitis C Infection China Hcv Test Kit Hcv Reagent Kit

Anti Hcv Cassette Linear
Cho Cells Anti Hepatitis C Virus Hcv Hcv Ns5b Antibody Abin

Jpma Journal Of Pakistan Medical Association

Anti Hcv Subtype 1b Ns5b Antibody Ab Abcam

Hepatitis C In Hemodialysis Units Diagnosis And Therapeutic Approach
Q Tbn And9gcsherkcbsj 4aouww9gobvir8nwnhwtd2demaxufhb 7hmcadt5 Usqp Cau

Hepatitis C Cancer Therapy Advisor

How To Test For Current Or Past Hcv Infection Hcv Exposure Choice Of Serological Assay And Testing Strategy Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
Http 158 232 12 119 Entity Hepatitis Publications Annex 6 1 Pdf
Www Fardavar Com Upload D9 85 D8 B4 D8 Ae D8 B5 D8 D8 D9 85 D8 Ad D8 B5 D9 D9 84 Anti hcv Pdf

Fig 3 Single Anti Hcv Test Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf

Anti Hcv Subtype 1b Ns5b Antibody 10d6 Ab1005 Abcam



