Real Time Release Testing
The MycoTOOL Mycoplasma RealTime PCR Test is a mycoplasma detection kit accepted for biopharmaceutical release testing by the FDA, EMA and further authorities for fast, easy, and reliable Mycoplasma testing Detect over 140 Mollicute species including Spiroplasma and Acholeplasma using universal primer designs Sensitivity you can count on.

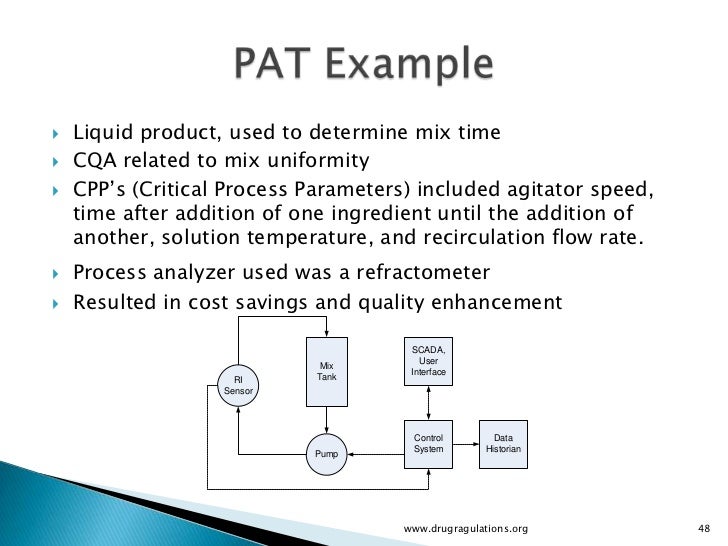
Real time release testing. Note Real Time Release Testing does not mean less testing!. Realtime cytolysis potency assays and derivation of KT 50 values In order to assess cytolysis of target cells as a function of time and effector cell number using the RTCA potency assay, nuclear red PC3 cells were treated with NK92 at ET ratios ranging from 101 to using quadruplicate wells. Spectroscopy tools like NIR or Raman are commonly used in process control and real time testing of pharmaceutical products using multivariate analysis methods Taking into consideration a difference between multivariate and traditional methods, the current approach of Q2 (R1) is not sufficient to establish the suitability of multivariate methods.
Such terms are often used differently by different companies or even different groups within a company I give you my view on the subject Release testing is testing a new version of a software (project or product) to verify, that the software can. The test kit is called the CDC 19 Novel Coronavirus (19nCoV) RealTime Reverse Transcriptase (RT)–PCR Diagnostic Panel On February 3, , CDC submitted an EUA package to expedite FDApermitted use of the CDC diagnostic panel in the United States. Agile Test Plan Agile test plan includes types of testing done in that iteration like test data requirements, infrastructure, test environments, and test results Unlike the waterfall model, in an agile model, a test plan is written and updated for every release Typical test plans in agile includes.
Real Time Release Testing – Has its Time Come?. 511 After completion of entry, Quality Control Section Incharge/Executive will fill the details in the ‘Prior to packing release slip in triplicate and release the product for packing 512 QC Section Incharge/Executive will send a yellow and green copy to production Dept and attach the third white copy with the testing report. For example, Biogen plans to use this distributed QC method of realtime release and review by exception in its new manufacturing facility near Solothurn, Switzerland When production starts up in 19, the Solothurn facility will achieve rawmaterial control through screening and genealogy, with minimal testing using rapid identification and.
Real Time Pain Relief’s flagship product, ORIGINAL PAIN Relief Cream, was created to solve this problem This fastacting pain relief cream is infused with 17 of nature’s ingredients, including aloe vera, willow bark, MSM, calendula, and more, and it has just the right amount of the active ingredient menthol to effectively relieve pain. Sample test questions are small subsets of test questions released from the STAAR test banks These test questions may have been previously administered A test form is a set of released test questions previously administered together to Texas students which reflects the STAAR test blueprints. A method for predicting dissolution profiles of directly compressed tablets for a fixed sustained release formulation manufactured in a continuous direct compaction (CDC) system is presented The methodology enables realtime release testing (RTRt).
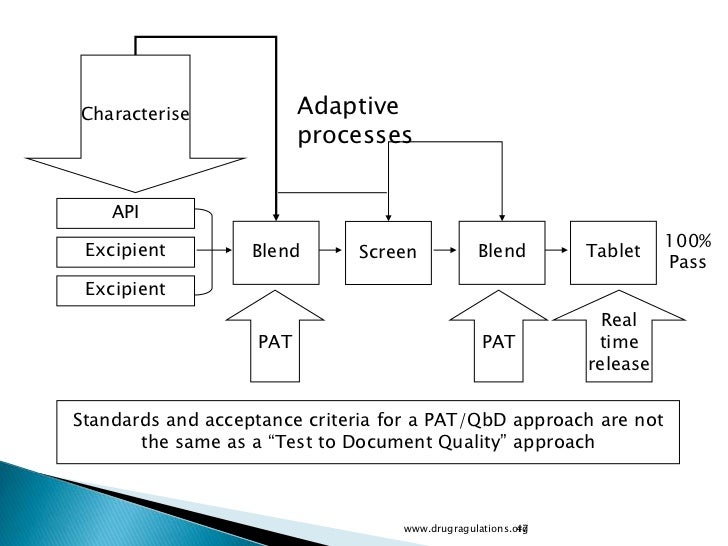
(for realtime studies) Climatic zone °C2 °C MKT3 % RH4 °C % RH I 0 0 42 21 45 II 216 2 52 25 60 III 264 279 35 30 35 IV 267 274 76 30 70 1 Based on Grimm W Storage conditions for stability testing in the EC, Japan and USA;. Dissolution Modeling for Real Time Release Testing (RTRT) Hanlin Li, Justin Prichard, Kelly A Swinney ©16 Vertex Pharmaceuticals Incorporated Outline • Introduction to continuous manufacturing and RTRT at Vertex • RTRT for dissolution – model development approach. Realtime release testing (RTRT) is defined as "the ability to evaluate and ensure the quality of inprocess and/or final drug product based on process data, which typically includes a valid combination of measured material attributes and process controls" (ICH Q8 R2) This article discusses sensors (process analytical technology, PAT) and control strategies that enable RTRT for the spectrum of critical quality attributes (CQAs) in biopharmaceutical manufacturing.
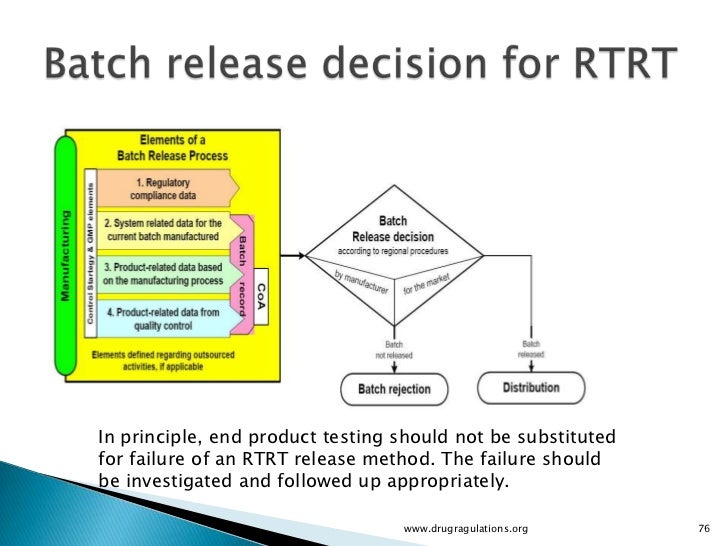
Real time release testing (RTRT) 19 31 Under RTRT, a combination of inprocess monitoring and controls may provide sufficient 21 evidence to justify batch release without the tests being repeated on a sample of the finished 22 product 23. Real Time Scenarios in Software Testing (Manual and Automated Testing) 1) Automated Test Case Scope is High than Manual Test Case Generally, in a Manual Test Case we insert/use one or two verification points only, because human user can’t concentrate on multiple verification points at a time during Test Execution. What is a RealTime Operating System (RTOS)?.
Real time processing deals with streams of data that are captured in realtime and processed with minimal latency to generate realtime (or nearrealtime) reports or automated responses For example, a realtime traffic monitoring solution might use sensor data to detect high traffic volumes This data could be used to dynamically update a map. At the 09 Emerson Users Exchange, Ali Afnan, senior Fellow at FDA's Center for Drug Evaluation and Research, discusses real time release, highlighting Aven. CDC’s new lab test is a “realtime” reverse transcription polymerase chain reaction, or rRTPCR, and it identifies all strains of EVD68 that we have been seeing this summer and fall The new test has fewer and shorter steps than the test that CDC and some states were using for the EVD68 outbreak.
In early , CDC developed its first laboratory test kit for use in testing patient specimens for SARSCoV2 The test kit is called the CDC 19 Novel Coronavirus (19nCoV) RealTime Reverse Transcriptase (RT)–PCR Diagnostic Panel On February 3, , CDC submitted an EUA package to expedite FDApermitted use of the CDC diagnostic panel in the United States. Four medical professionals with COVID19 who met the criteria for hospital release or lifting of quarantine in China had positive realtime reverse transcriptasepolymerase chain reaction (RTPCR) results 5 to 13 days later, according to a research letter published yesterday in JAMA The researchers said the results suggest that current criteria for hospital release or lifting of quarantine. Always keep in mind, not to leave any stone unturned when it comes to testing your application It’s time to formulate a test strategy Create a matrix of the environment so that the software is tested on all platforms Like, Windows 10Internet Explorer 11 Windows Office 10 Like Android 422 Chrome browser.
SAN FRANCISCO, Sept 24, /PRNewswire/ United Airlines today became the first US airline to launch a COVID19 pilot testing program for travelers that could make it easier for them to manage quarantine requirements and entry conditions of popular destinations around the world Starting on October 15, customers traveling on United from San Francisco International Airport (SFO) to. It means realtime testing Example Testing includes the realtime scenario, it also involves the scenarios based on the experience of the testers sanity testing is also known as build version testing or build acceptance testing this is the first test conducted after every build release to ensure that any functional changes occurred. Realtime release testing can be defined as a set of inprocess controls that may provide greater assurance of product quality than endproduct testing.
A sample in the test set is identified as a qualified sample when its MDq is less than MDu;. Real Time Release Testing (RTRT) the ability to evaluate and ensure the quality of inprocess and/or final product based on process data, typically include a valid combination of measured. Real Time Release Testing guideline 7 Westferry Circus Canary Wharf London E14 4HB United Kingdom Telephone 44 (0) 7418 8400 Facsimile 44 (0) 7418 8416 Email info@emaeuropaeu Website wwwemaeuropaeu An agency of the European Union © European Medicines Agency, 12 Reproduction is authorised provided the source is acknowledged.
Realtime operating system (RTOS) is an operating system intended to serve real time application that process data as it comes in, mostly without buffer delay The full form of RTOS is Real time operating system In a RTOS, Processing time requirement are calculated in tenths of seconds increments of time. The test is a realtime RTPCR test intended for the qualitative detection of nucleic acids from SARSCoV2 in nasopharyngeal and oropharyngeal swab samples from patients who meet the CDC SARSCoV2 clinical criteriaThe test runs on the cobas 6800/00 Systems and has a fullprocess negative control, positive control and internal control. As a project manager or project lead, sometimes you might face a situation to call off the testing to release the product early In those cases, you have to decide whether the testers have tested the product enough or not There are many factors involved in the real time projects to decide when to stop testing.
GMP lot or batch release testing services for biologic drug substances or drug products are important to ensure the quality control of proteins, monoclonal antibodies (mAbs) or biosimilars A range of tests are required as part of release testing activities to address the purity, concentration, consistency, identity and biosafety of products. DOI 1072/TRN Corpus ID Tools for real time release testing (RTRt) in batch and continuous tablet manufacturing @inproceedings{Pawar16ToolsFR, title={Tools for real time release testing (RTRt) in batch and continuous tablet manufacturing}, author={P Pawar}, year={16} }. Last November, Spotify confirmed it was testing realtime lyrics synced to music in select markets Tomorrow, the company will announce the launch of its new lyrics feature in 26 worldwide markets.
Sample test questions are small subsets of test questions released from the STAAR test banks These test questions may have been previously administered A test form is a set of released test questions previously administered together to Texas students which reflects the STAAR test blueprints. Scope 11 This practice establishes an approach to the realtime release testing (RTRT) of pharmaceutical water based on the total organic carbon (TOC) attribute using online total organic carbon (OLTOC) instrumentation that is in agreement with current regulatory thinking 12 This practice is harmonized with or supports the concepts of relevant ASTM International Committee E55 on Manufacture of Pharmaceutical Products standards, ICH Harmonized Tripartite Guidelines, the US FDA PAT. Yokogawa PAT Solution to RealTime Release Testing (RTRT) Yokogawa's approach to the development of process analytical technology (PAT) solutions relies on realtime monitoring of critical quality attributes (CQA) to achieve lean manufacturing Online quality attributes can be directly monitored by means of near infrared (NIR) analysis.
BLUE BELL, Pa, August 24, – Unisys Corporation (NYSE UIS) today announced a relationship with Inspire Health Alliance, a USbased healthcare company, to support Inspire's offer of realtime COVID19 registration, testing and access control that is both cost effective and scalable to organizations of any size. By James Davidson, PhD Nov 17, 15 Compliance FDA Generics Regulatory Affairs Generally, pharmaceutical manufacturing involves laboratory testing on product sampled at the end of the manufacturing process to assure the product quality as part of the product release. Dissolution Modeling for Real Time Release Testing (RTRT) Hanlin Li, Justin Prichard, Kelly A Swinney ©16 Vertex Pharmaceuticals Incorporated Outline • Introduction to continuous manufacturing and RTRT at Vertex • RTRT for dissolution – model development approach.
This realtime liposomal drug release assay allows for better understanding of the factors important in governing release of topotecan. The Panther Fusion SARSCoV2 assay is a realtime RTPCR in vitro diagnostic test intended for the qualitative detection of RNA from the SARSCoV2 isolated and purified from nasopharyngeal (NP) and oropharyngeal (OP) swab specimens obtained from individuals who meet COVID19 clinical and/or epidemiological criteria The Panther Fusion SARS. Agile Test Plan Agile test plan includes types of testing done in that iteration like test data requirements, infrastructure, test environments, and test results Unlike the waterfall model, in an agile model, a test plan is written and updated for every release Typical test plans in agile includes.
CurtissWright’s Defense Solutions division’s Aerospace Instrumentation (AI) group has recently added the popular IADS realtime and posttest display and analysis software to its product line – you can learn more hereIADS excellent product development and support will continue without interruption IADS is a Real Time and Post Test display and analysis software suite that supports. Calendar information provides schedules of release trains, application releases, environments, and turnovers Timeline views provide realtime actual vs plan status Release dashboard provides rapid insight into the entire release management lifecycle Outofthebox tracking and process improvement KPIs. Real‐time release testing (RTRT) is defined as “the ability to evaluate and ensure the quality of in‐process and/or final drug product based on process data, which typically includes a valid combination of measured material attributes and process controls” (ICH Q8R2).
Real Time Release Testing Controls Blend uniformity assured in blending step (online NIR spectrometer for blending endpoint) API assay is analysed in blend by HPLC API content could be determined by online NIR, if stated in filing Tablet weight control with feedback loop in compression step No end product testing for Assay and Content Uniformity (CU) Would pass finished product specification for Assay and Uniformity of Dosage Units if tested because assay assured by combination of blend. Realtime software systems have strict timing constraints To test if timing constraints are met, realtime testing is used Usability testing System test Postrelease Time introduced Requirements 1× 3× 5–10× 10× 10–100× Architecture – 1× 10× 15× 25–100× Construction – – 1× 10×. Real Time Release Testing ‘The ability to evaluate and ensure the quality of inprocess and/or final product based on process data, which typically include a valid combination of measured material attributes and process controls’ (ICH Q8(R2)) 3.
Sample test questions are small subsets of test questions released from the STAAR test banks These test questions may have been previously administered A test form is a set of released test questions previously administered together to Texas students which reflects the STAAR test blueprints. Otherwise it is identified as an unqualified sample Therefore, the qualitative releasing criterion of R paridis is MDq < MDu 342 Real time release testing The established NIR models were then applied to RTRT of R paridis. Real time release testing (RTRT) 19 31 Under RTRT, a combination of inprocess monitoring and controls may provide sufficient 21 evidence to justify batch release without the tests being repeated on a sample of the finished 22 product 23.
Enabling Real Time Release testing (RTRt) with NIRbased prediction of dissolution for Tablets made by Continuous Direct Compression (CDC) G Drazer, P Pawar, Y Wang, G Keyvan, G Callegari A Cuitino and F Muzzio Rutgers, The State University of New Jersey. The main advantage of RTRT is simply business efficiency Realtime assurance of quality, and the avoidance of storage or quarantine of intermediates and drug product while awaiting test results, will enable much greater efficiency throughout the process. Realtime cytolysis potency assays and derivation of KT 50 values In order to assess cytolysis of target cells as a function of time and effector cell number using the RTCA potency assay, nuclear red PC3 cells were treated with NK92 at ET ratios ranging from 101 to using quadruplicate wells.
Abstract A comprehensive commercial control strategy for tablet content and content uniformity focussed on the unit operation of compression is presented and is proposed to enable real time release for these critical quality attributes The control strategy is based on process understanding, process control through compaction force weight control on the tablet press, periodic checks of mean and individual tablet weight combined with atline testing of tablet content by near infrared (NIR). LinkSēq is the first commercially available HLA testing platform developed for use with RealTime PCR instrumentation that allows the identification of all classical HLA genes in a single test. It means realtime testing Example Testing includes the realtime scenario, it also involves the scenarios based on the experience of the testers #18) Exploratory Testing Exploratory Testing is informal testing performed by the testing team.
This realtime liposomal drug release assay allows for better understanding of the factors important in governing release of topotecan The assay will be essential towards designing liposomal formulations of topotecan (and potentially of other camptothecin derivatives such as irinotecan) with optimized retention times and better therapeutic. This document outlines the requirements for applications that propose real time release testing for active substances, intermediates and finished products It addresses the need for interaction between quality assessors and good manufacturing practice inspectors in the approval process This guideline is a revision of the guideline on parametric release and does not introduce new requirements. 24 Specifications for ‘Large n’ UDU testing Specifications based on zero tolerance* problematic when testing large samples (ie >30) ‘Large n Counting Test’** Analyze X samples Assess number of samples (n) outside.
The most important market for drug products.

Real Time Release Testing

Manufacturing Implementation And The Pharmaceutical Quality System Ppt Video Online Download
2
Real Time Release Testing のギャラリー

Experts Real Time Release Testing Not Primary Goal Of Qbd

Figure 2 2 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar

Real Time Release Testing
2

Recording Webinar Revised Annex 17 What S New Real Time Release Testing Rtrt Challenges And Opportunities Eca Academy

The Future Of Quality Control
Www Ema Europa Eu En Documents Presentation Presentation Medicines Healthcare Products Regulatory Agency Mhra Overview Applications Marketing En Pdf

The Potential Application Of Real Time Release Testing For The Biomanufacture Of Autologous Cell Based Immunotherapies Ip Asset Ventures

Ema Approves Janssen S Prezista Continuous Manufacturing Line

Moving Toward Real Time Release Testing Pharmaceutical Technology

Real Time Release Testing Of Pharma Drug Products Full Webinar Pharmaceutical Report

Biophorum Collaboration For Improving Biopharmaceutical Manufacturing

Us Fda Offers More Perspective On Real Time Release Testing
Terahertz Real Time Release Testing On Vimeo
2

Pharmaceutical Technology Europe March 12

Single Use Enabled Automated Continuous Monoclonal Antibody Production With Real Time Release 4th International Symposium On Continuous Manufacturing Of Pharmaceuticals

Enabling Real Time Release Testing By Nir Prediction Of Dissolution Of Tablets Made By Continuous Direct Compression Cdc Sciencedirect

Real Time Release Testing

Pdf Opportunities And Challenges Of Real Time Release Testing In Biopharmaceutical Manufacturing
Real Time Release Testing

Astm E2656 Process Control And Real Time Release Testing Using Total Organic Carbon Ge Analytical Instruments Pdf Catalogs Technical Documentation Brochure

Practical Considerations For Implementation Of Real Time Release

Quality Implementation Working Group On Q8 Q9 And Q10 Questions Answers Business Evaluation

Review Of Real Time Release Testing Of Pharmaceutical Tablets State Of The Art Challenges And Future Perspective Sciencedirect
Real Time Release Testing
Rtrt Real Time Release Testing

Control Strategy And A Real Time Release Testing From A Development Line To
Real Time Release Testing

Fillable Online Fda Regulatory Perspective On Real Time Release Testing Rtrt Fda Fax Email Print Pdffiller

Real Time Release Testing Of Tablets

The Potential Application Of Real Time Release Testing For The Biomanufacture Of Autologous Cell Based Immunotherapiesbioprocess International

Case Study Ppt Download

Astm E2656 16 Red Standard Practice For Real Time Release Testing Of Pharmaceutical Water For The Total Organic Carbon Attribute Standard Redline Pdf Bundle
Http Www Journalofpharmaceuticalresearch Org Index Php Kpc Article Download 1114
2
2
Http Www Gmp Compliance Org Guidemgr Files 18 09 11 Annex 17 De Final Pdf
Depts Washington Edu Cpac Activities Meetings Satellite 10 Thursday Cook implementation of rtrt cpac rome 10 3 25 Pdf
Casss Site Ym Com Resource Resmgr Cmc No Am Jul Spkr Slds 15 Cmcs Clarkeliana Pdf

Pdf Opportunities And Challenges Of Real Time Release Testing In Biopharmaceutical Manufacturing

Real Time Release Testing Of Tablet Content And Content Uniformity Sciencedirect
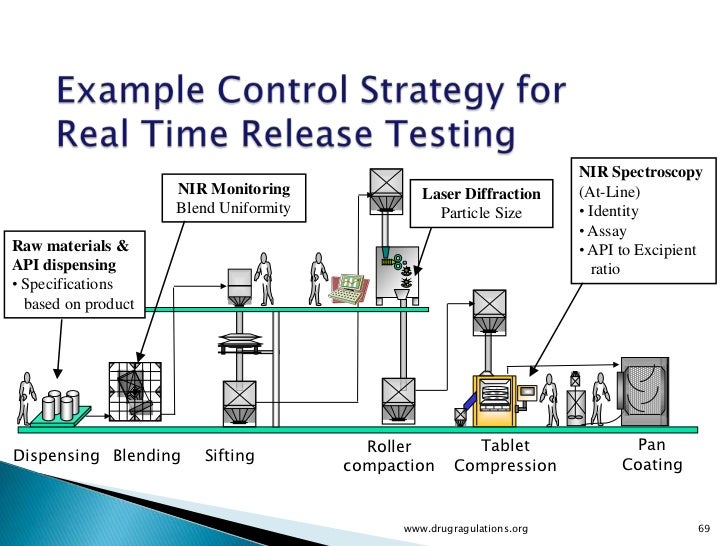
Real Time Release Testing

Pdf Opportunities And Challenges Of Real Time Release Testing In Biopharmaceutical Manufacturing

Figure 1 3 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar

The Potential Application Of Real Time Release Testing For The Biomanufacture Of Autologous Cell Based Immunotherapiesbioprocess International

Review Of Real Time Release Testing Of Pharmaceutical Tablets State Of The Art Challenges And Future Perspective Sciencedirect

Pharmaceutical Microbiology Revised Annex 17 On The Real Time Release Testing

A Case Study In Real Time Release Testing
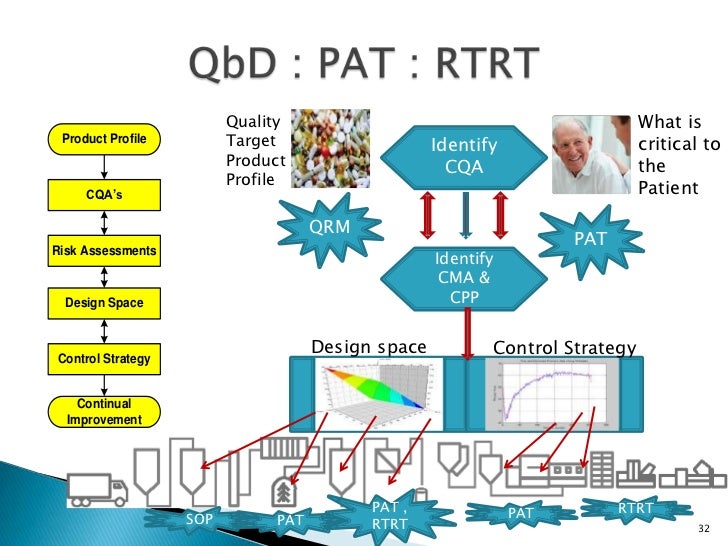
Real Time Release Testing

Quantitative Real Time Release Testing Of Rhubarb Based On Near Infrared Spectroscopy And Method Validation Sciencedirect

Figure 2 1 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar
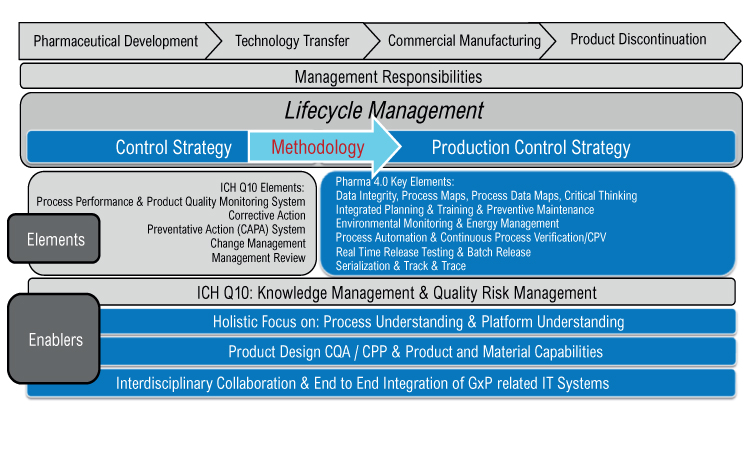
A Holistic Approach To Production Control Pharmaceutical Engineering

An Approach Combining Real Time Release Testing With Near Infrared Spectroscopy To Improve Quality Control Efficiency Of Rhizoma Paridis Sciencedirect

Development And Evaluation Of Accelerated Drug Release Testing Methods For A Matrix Type Intravaginal Ring Sciencedirect
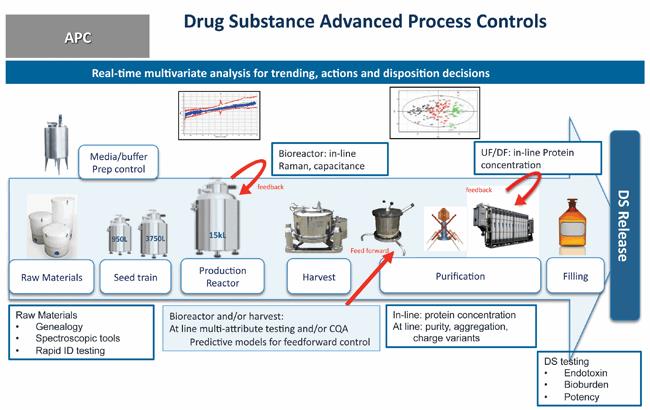
A Case Study In Real Time Release Testing

Real Time Release Testing A New Quality Paradigm For Pharmaceutical Development By Maithreye Dasu J Ram Naresh India Digital Copy Link

Astm E2656 Process Control And Real Time Release Testing Using Total Organic Carbon Ge Analytical Instruments Pdf Catalogs Technical Documentation Brochure

Real Time Release Testing
Http Pqri Org Wp Content Uploads 15 08 Pdf Thurau Pdf

Mapping Future Technology Needs For Real Time Release Testing

Astm E2656 10 Standard Practice For Real Time Release Testing Of Pharmaceutical Water For The Total Organic Carbon Attribute

Mapping Future Technology Needs For Real Time Release Testing May 22 19 9 00 Am Usa

Real Time Release Testing
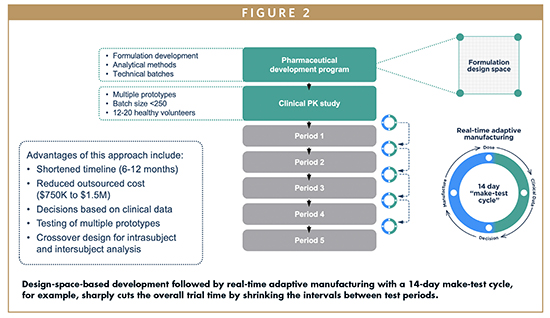
Modified Release Alternative Strategies For Development Of Modified Release Dosage Forms

Daniel Markl Research Group Website Overview
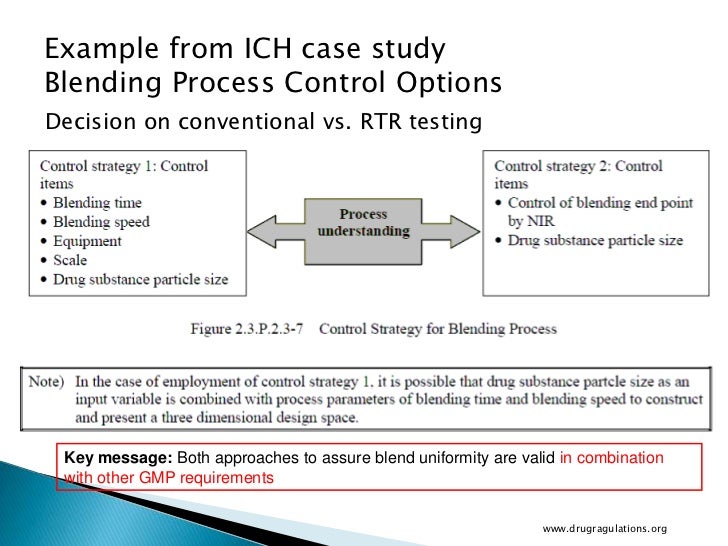
Real Time Release Testing

In Line Monitoring Real Time Release Testing In Biopharmaceutical Processes Prioritization And Cost Benefit Analysis Biophorum
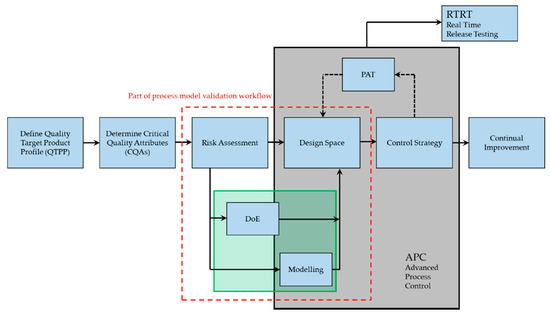
Processes Free Full Text Digital Twin For Lyophilization By Process Modeling In Manufacturing Of Biologics

Digitization Automation And Online Testing In Pharma Binocs
Www Casss Org Resource Resmgr Cmc Japan 18 Cmcjp Grosssteffen Pdf
Www Casss Org Resource Resmgr Cmc Euro Speaker Slides Cmc Europe 19 19 Jekerle Veronika Pp Sli Pdf

Automating Android Build Test Release Cycle By Sanyam Garg Truebil Engineering Medium

Mapping Future Technology Needs For Real Time Release Testing

Real Time Release Testing

European Commission Brussels Date

4 Real Time Release Testing

Figure 1 2 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar
Www Ema Europa Eu En Documents Scientific Guideline Guideline Real Time Release Testing Formerly Guideline Parametric Release Revision 1 En Pdf

Browsing Theses And Dissertations All Schools By Subject Real Time Release Testing

Product Real Time Release For The Microbial Critical Quality Attribute Using Qbd Approach American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology

Guideline Real Time Release Testing Formerly Guideline Parametric Release Revision 1 En Sterilization Microbiology Environmental Monitoring

Real Time Release Testing Of Tablets

Robotic Microplate Voltammetry For Real Time Hydrogel Drug Release Testing Sciencedirect
Real Time Release Testing For Dissolution Has Arrived Ips

Real Time Release Testing Has Its Time Come Lachman Consultant Services Inc
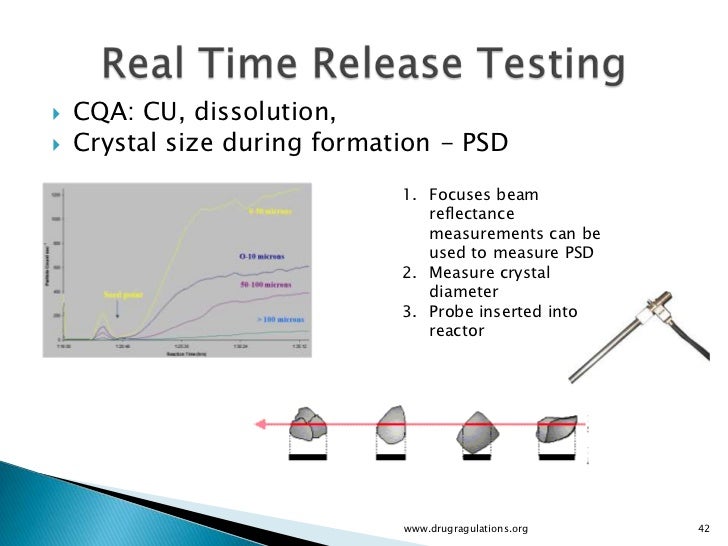
Real Time Release Testing

Ema Extends Real Time Release Testing Role In New Guidelines
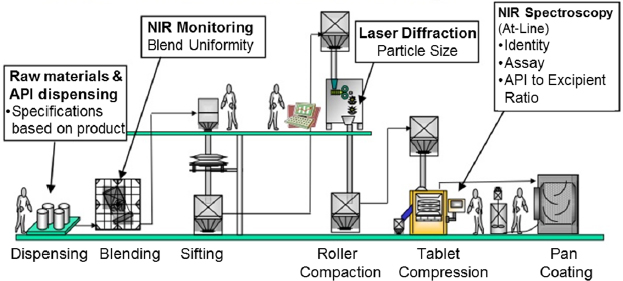
Blog Real Time Release Testing
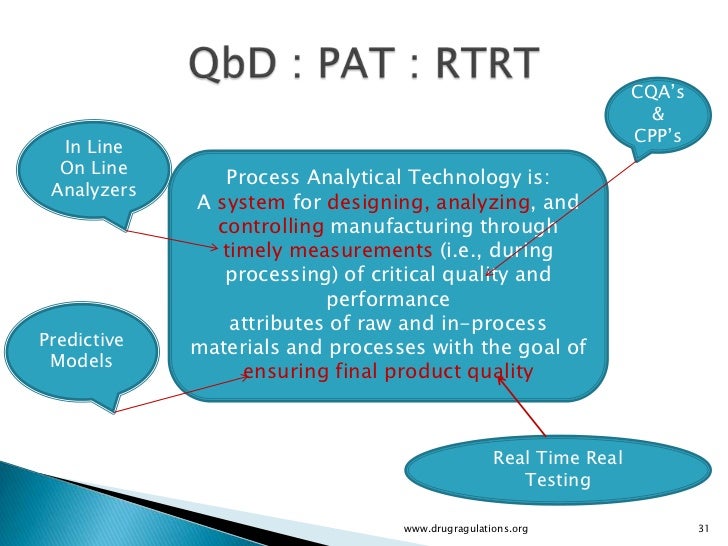
Real Time Release Testing
Www Pearrl Eu Uploads 7 0 9 9 11 Hpra Pat And Continuous Manufacturing Final For Informationwm Pdf

Real Time Release Testing
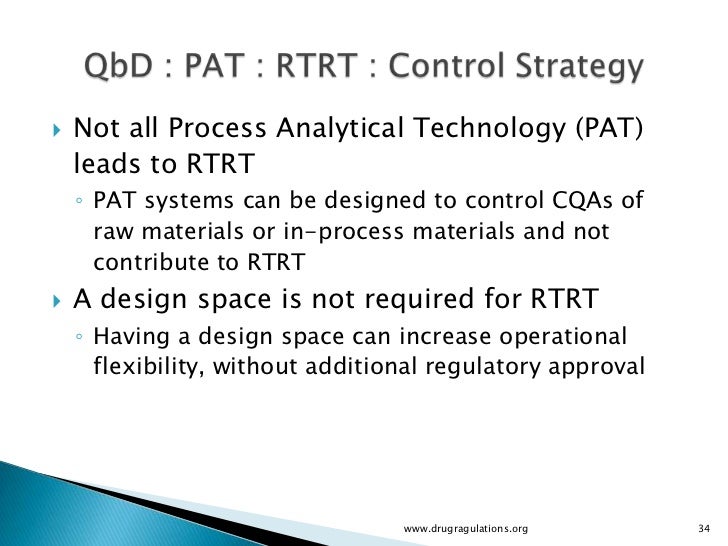
Real Time Release Testing

Figure 1 1 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar
2

Guideline On Real Time Release Testing Ipq
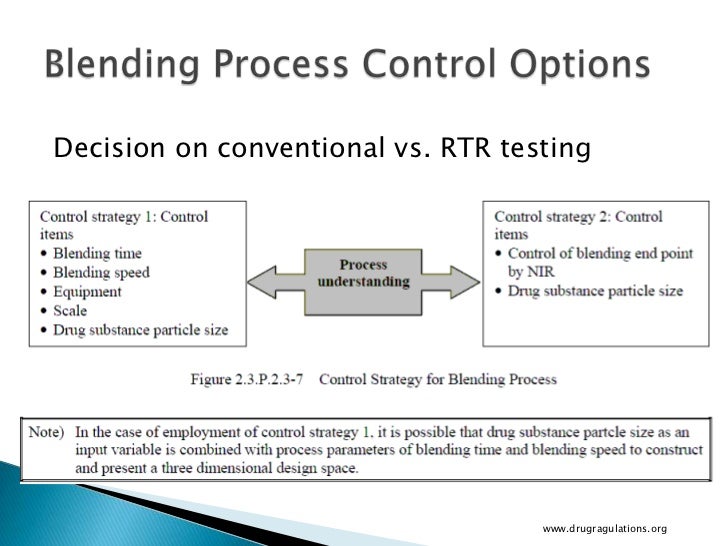
Real Time Release Testing



