Iso 13485 2003
Equivalence ISO Superceding Superceded by LEGALLY BINDING DOCUMENT Step Out From the Old to the NewJawaharlal Nehru Invent a new India using knowledgeSatyanarayan Gangaram Pitroda Addeddate 1744 Identifier govinisiso Identifierark.

Iso 13485 2003. The ISO standard was updated for two main reasons to keep up with changes in the industry and to address changes in the underlying ISO 9001 standard While the old ISO 03 standard was based on the old ISO 9001 00 standard, the new one is based on ISO 9001 08. ISO /Cor 109 Medical devices — Quality management systems — Requirements for regulatory purposes — Technical Corrigendum 1 This standard has been revised by ISO General. ISO Requirements Establishment of a quality management system for medical devices A manufacturer must have quality procedures that are documented, controlled, and effectively implemented and maintained Ensuring that personnel have the right experience, education, training, and skills.
ISO is a standalone QMS standard, derived from the internationally recognized and accepted ISO 9000 quality management standard series ISO adapts the ISO 9000 processbased model for a regulated medical device manufacturing environment While ISO is based on the ISO 9001 process model concepts of Plan, Do, Check, Act, it is designed for regulatory compliance. We are global, we’re independent and we’re a trusted service provider to 80,000 businesses. This third edition of ISO cancels and replaces the second edition (ISO ) and ISO/TR , which have been technically revised It also incorporates the Technical Corrigendum ISO /Cor109 A summary of the changes incorporated into this edition compared with the previous edition is given in Annex A.
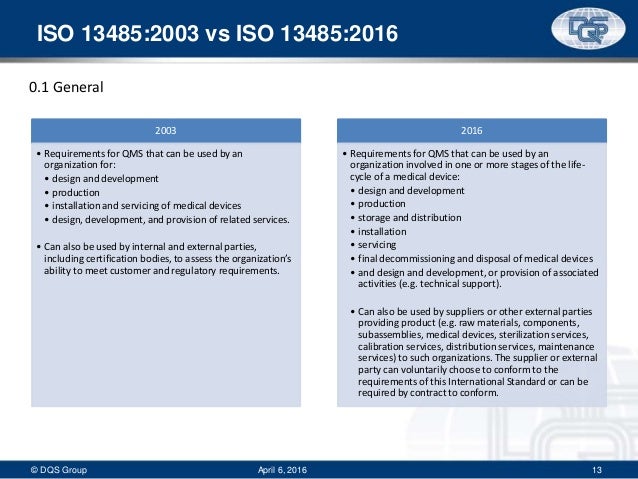
ISO promotes a process approach when developing, implementing, and improving a QMS Requirements from Customers & Regulatory Authorities Valueadded activities Information Flow Slide 5 of 86 ISO – An Overview (KL, Malaysia, March 08) Gunter Frey & Hideki Asai. ISO ISO was released in March 16 All companies have 3 years in which to transition There are few industries where the importance of product conformity is as crucial as the design, manufacture, and testing of medical devices. ISO clause 41 states, “Where an organization chooses to outsource any process that affects product conformity with requirements, the organization shall ensure control over such processes Control of such outsourced processes shall be identified within the quality management system”.
ISO (E) PDF disclaimer This PDF file may contain embedded typefaces In accordance with Adobe's licensing policy, this file may be printed or viewed but shall not be edited unless the typefaces which are embedded are licensed to and installed on the computer performing the editing. ISO Medical devices Quality management systems Requirements for regulatory purposes is an International Organization for Standardization (ISO) standard published for the first time in 1996;. This third edition of ISO cancels and replaces the second edition (ISO ) and ISO/TR , which have been technically revised It also incorporates the Technical Corrigendum ISO /Cor109 A summary of the changes incorporated into this edition compared with the previous edition is given in Annex A.
ISO ISO is the quality management system standard for medical device manufacturers ISO is based on ISO 9001 and supplemented with additional quality management requirements relating to design, special processes, environmental control, traceability, documentation, and regulatory actions. ISO is the main Quality Management System (QMS) standard for medical devices, although several countries have their own set of regulations As an example, the United States plans to harmonize the Food and Drug Administration (FDA) requirements for medical devices with ISO. ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services The primary objective of ISO is to facilitate harmonized medical device regulatory requirements for quality management systems.
Get ISO registration & compliance information for the ISO , ISO Standard DAC Audit Services, an ISO Audit Provider. ISO promotes a process approach when developing, implementing, and improving a QMS Requirements from Customers & Regulatory Authorities Valueadded activities Information Flow. ISO is an ISO standard, published in 03, that represents the requirements for a comprehensive management system for the design and manufacture of medical devices This standard supersedes earlier documents such as EN and EN (both 1997), the ISO published in 1996 and ISO 134 (also 1996).
ISO is an ISO standard, published in 03, that represents the requirements for a comprehensive management system for the design and manufacture of medical devices This standard supersedes earlier documents such as EN and EN (both 1997), the ISO published in 1996 and ISO 134 (also 1996) While it remains a standalone document, ISO is generally harmonized with ISO 9001. Division Name Medical Equipment and Hospital Planning Section Name Hospital Planning (MHD 14) Designator of Legally Binding Document IS/ISO Title of Legally Binding Document Medical DevicesQuality Management SystemsRequirements for Regulatory Purposes Number of Amendments Equivalence ISO. ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services.
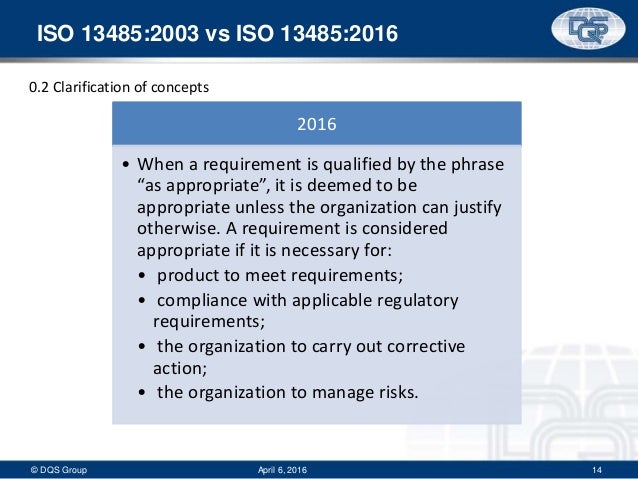
The ISO standard was updated for two main reasons to keep up with changes in the industry and to address changes in the underlying ISO 9001 standard While the old ISO 03 standard was based on the old ISO 9001 00 standard, the new one is based on ISO 9001 08. Clause 12 Application of ISO permits Manufacturers of Class II medical devices to exclude clause 73 Design and development from the QMS, with justification(s) ISO recognizes that some requirements in Clause 7 may be "not applicable" due to the nature of the medical device. The complete process is subject to a quality management system with comprehensive control and documentation requirements which is now certified according to ISO Regular quality and customer audits as well as the annual surveillance by the certification body ensure the control and continuous improvement of the quality management system.
ISO (E) © ISO 03 — All rights reserved v 0 Introduction 01 General This International Standard specifies requirements for a quality management system that can be used by an organization for the design and development, production, installation and servicing of medical devices, and. Iso 03 ระบบการบริการคุณภา พการผลิตเครื่องมือแพทย์ 2 ISO 07 การบริหารความเสี่ยงเครื่องมือแพทย์. ISO represents the requirements for a comprehensive management system for the design and manufacturing of medical devices including Controls to ensure product safety Risk management activities Inspection and traceability Documentation and validation of processes Verification of the effectiveness of corrective and preventive actions.
It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devicesThis standard supersedes earlier documents such as EN (1993. ISO = ISO Medical Device Requirements ISO Medical Devices Quality Management System requirements for regulatory purposes is an ISO standard, originally published in 1996 This standard incorporated aspects of ISO Quality Management System, but is specific to the global medical device industry. Equivalence ISO Superceding Superceded by LEGALLY BINDING DOCUMENT Step Out From the Old to the NewJawaharlal Nehru Invent a new India using knowledgeSatyanarayan Gangaram Pitroda Addeddate 1744 Identifier govinisiso Identifierark.
Issued by the International Organization for Standardization (ISO), the ISO standard is an effective solution to meet the comprehensive requirements for a QMS in the medical device industry Adopting ISO provides a practical foundation for manufacturers to address the EU Medical Device Directive (MDD), the EU Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to the safety and quality of medical devices. The design and development of these devices is part of the manufacturer’s quality management system (QMS) and ISO —“Medical devices—Quality management systems—Requirements for regulatory purposes” provides requirements for the design and development process, including the incorporation of risk management. ISO was finally revised after 13 years and has many significant changes The three main reasons for the updates were The medical device regulatory environment has evolved since 03 Risk management and riskbased decisionmaking processes have become the main focus of the entire medical device industry;.
Unlike ISO , ISO stresses the safety and efficacy of medical devices that are being produced For this reason risk management is an essential process that needs to be adopted into the ISO quality management system. ISO has no explicit criteria to describe requirements of transferring a product from design and development to production ISO corrects this and includes explicit requirements The design and development transfer addition actually strengthens the similarities with FDA with respect to design controls / design and development. I would like to share eleven clauses that have significantly changed in ISO from ISO and how these changes relate to FDA 21 CFR Part 0 1 ISO CLAUSE 4 QUALITY MANAGEMENT SYSTEM & 41 GENERAL REQUIREMENTS The biggest change of these clauses against ISO is the 16 version requires application of a.
Corresponding clause in ISO Cleanliness of product and contamination control In the new version of the standard, this clause is extended and the organizations are required to identify products that cannot be cleaned prior to sterilization or use. ISO an international standard designed to orient quality management systems toward the design, development, production, and installation of medical devices and related services Medical Devices are products designed to cope with disease, treat injuries, investigate and augment human functions and they also are made up of a host of other apparatuses and appliances. ISO represents the requirements for a comprehensive management system for the design and manufacturing of medical devices including Controls to ensure product safety Risk management activities Inspection and traceability Documentation and validation of processes Verification of the effectiveness of corrective and preventive actions.
It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices This standard supersedes earlier documents such as EN (1993 and 1996) and EN (1996), the previously published ISO (1996 and 03), and ISO 134. ISO is a certification intended for organizations that provide medical devices The standard puts an emphasis on regulatory requirements, custom requirements, risk management and maintaining effective processes such as safe design, manufacture and distribution of medical devices. Unfortunately, corrections on the ISO took a long time The new version of ISO was ready to be released in 16 although it relied on ISO So, when ISO was finally released with the new structure, was also already ready for release with the ISO structure.
ISO January 10, 21 The design, manufacture and distribution of InVitro Diagnostics and products of cell culture, molecular biology and microbiology BSI MD Life Technologies Holdings Pte Ltd Blk 33 Marsiling Industrial Estate Rd 3 #07–06 Singapore ISO EN ISO November 07, 21. ISO Medical devices Quality management systems Requirements for regulatory purposes is an International Organization for Standardization (ISO) standard published for the first time in 1996;. The design and development of these devices is part of the manufacturer’s quality management system (QMS) and ISO —“Medical devices—Quality management systems—Requirements for regulatory purposes” provides requirements for the design and development process, including the incorporation of risk management In addition, the standard covers the analysis of feedback from experience during the use phase and the conducting of postmarket surveillance.
ISO becomes obsolete on March 1, 19 but companies should begin the transition process as soon as possible There are many differences between the versions but the changes are easily implemented with AlvaMed’s help and support. ISO Certification is recognized as a worldwide quality certification specific to the Medical Device industry According the International Organization for Standardization, "ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and. ISO ISO > Overview ISO is the quality management system standard for medical device manufacturers ISO is based on ISO 9001 and supplemented with additional quality management requirements relating to design, special processes, environmental control, traceability, documentation, and regulatory actions Unlike ISO.
It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devicesThis standard supersedes earlier documents such as EN (1993. ISO/TR ), Medical devices — Quality management systems — Guidance on the application of ISO 13 ISO , Medical devices — Application of risk management to medical devices 14 ISO , Guidelines for quaity and/or environmental management systems auditing 15. The standard Under ISO , if you perform the function, the process will be audited The first difference encountered between ISO and ISO is in the quality management system The former standard deletes the words “continually improve” from the requirement to establish and maintain a quality management system.
ISO (Quality Management System for Medical Devices) ISO , based on the ISO Certification process model, suggests that the application and management of a system of processes is an effective way to ensure good quality management All requirements of this International Standard are specific to organizations providing medical devices, regardless of the type or size of the organization. ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services. ISO Medical devices Quality management systems Requirements for regulatory purposes is an International Organization for Standardization (ISO) standard published for the first time in 1996;.
Organizations dealing with the design, development, production, installation or servicing of medical equipment, devices and technology Why BSI?. ISO (Quality Management System for Medical Devices) ISO , based on the ISO Certification process model, suggests that the application and management of a system of processes is an effective way to ensure good quality managementAll requirements of this International Standard are specific to organizations providing medical devices, regardless of the type or size. ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services.
ISO = ISO Medical Device Requirements ISO Medical Devices Quality Management System requirements for regulatory purposes is an ISO standard, originally published in 1996 This standard incorporated aspects of ISO Quality Management System, but is specific to the global medical device industry. ISO ISO is the quality management system standard for medical device manufacturers ISO is based on ISO 9001 and supplemented with additional quality management requirements relating to design, special processes, environmental control, traceability, documentation, and regulatory actions. BS EN ISO replaces BS EN ISO which has been withdrawn Who should buy it?.
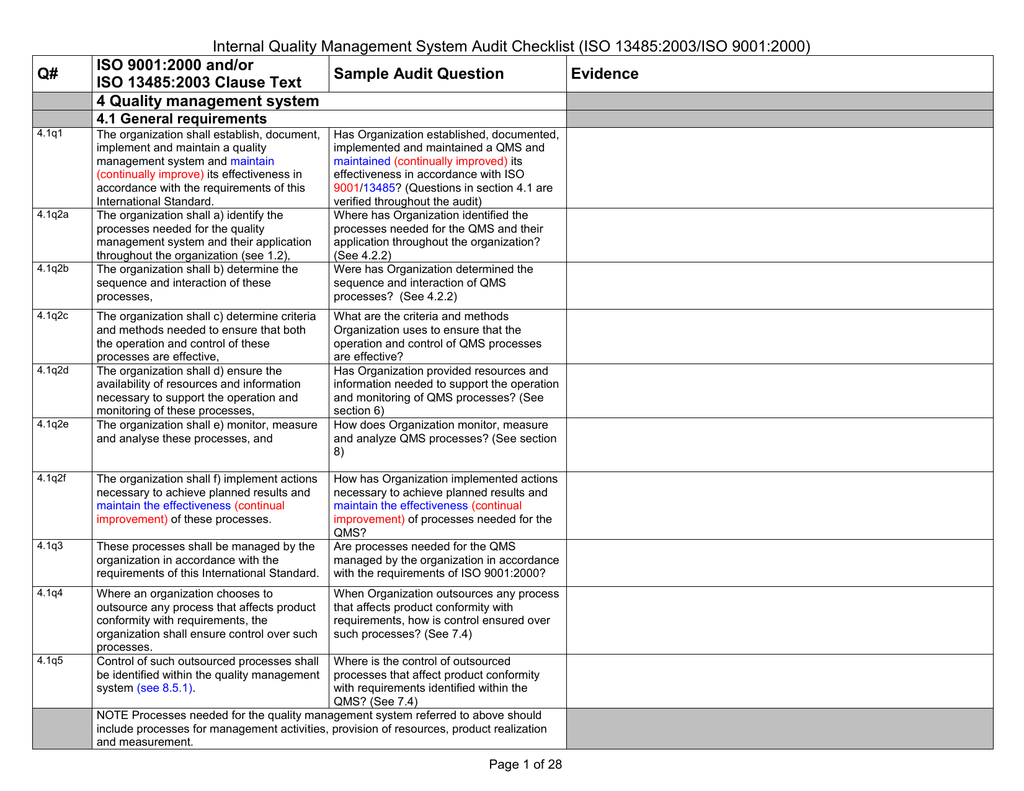
Currently, ISO and 16 will coexist which allows time for everyone to transition to the new standard ISO has an increased emphasis on regulatory requirements, risk management, validation/verification & design transfer, outsourced processes & supplier control, and feedback. ISO is a Management System Standard focused towards manufactures of Medical DevicesThe ISO Certificate was published by ISO in 03 The ISO standards outline a Comprehensive Management System for medical device manufacturers The ISO standard primarily facilitates medical device regulatory requirements that are. ISO Clause TextSample Audit QuestionEvidence 4 Quality management system 41 General requirements 41q1The organization shall establish, document, implement and maintain a quality management system and maintain (continually improve) its effectiveness in accordance with the requirements of this International Standard Has Organization established, documented, implemented and maintained a QMS and maintained (continually improved) its effectiveness in accordance with ISO.

Iso 03 Ppunch Ppunch

Yamaha Motor Jw Wheelchairs Division Acquires Iso 03 Yamaha Motor Co Ltd

Iso 03 Certificate 15 Philips Innovation Services
Iso 13485 2003 のギャラリー

Life Or Death In The Medical Industry Iso Matters Amax Blog And Insights

Sierra Labs Blog Iso 03

Iso 03 Certification Definition What Is Iso 03 Find Iso 03 Companies On Thomasnet Com Supplier Diversity Quality Discovery

Iso 03 Quality Management Certification Timestrip
Iso Euro Certification

En Iso 03 Ac 07 Medical Devices Quality Management Systems Requirements For Regulatory Purposes Iso 03

Iso 03 Finetech Research And Innovation Corporation

A Comprehensive Guide To Iso Nqa

Ansi mi Iso 03 Quality Management Systems Medical Devices System Requirements For Regulatory Purposes

Iso 03 Certificate 18 Philips Innovation Services

Iso Certification Medical Manufacturing John Evans Sons

Iso 03 Medical Device Quality Management Royalty Free Cliparts Vectors And Stock Illustration Image 7268

Lemo Usa Achieves Iso 03 Certification

Biologic Therapies Inc Achieves Iso 03 Certification

What Are The Major Differences Between Iso 03 And The Latest Version Iso 16 Regarding Risk Managment And Company Leadership

Ppt Transitioning From Iso 9001 08 To Iso 03 Going After Medical Device Business Powerpoint Presentation Id 6702

Iso 16 Certificate Iso 03 सर ट फ क शन सर व स आईएसओ 03 सर ट फ क शन सर व स आईएसओ 03 प रम णन स व ए In Nabha Patiala Revolutionary Consultants Id

Eclipse Aesthetics Receives Iso 03 Certification Biospace

Ortho Baltic Conforms To Iso 03 Baltic Implants

Iso 03 Certificate Medical Gas Fittings Bay Corp

Iso Audit Checklist Pdf Fill Online Printable Fillable Blank Pdffiller

Nuraleve Nordocs R D Facility Certified To The Iso 03 And Iso 9001 08

Iso 03 Certification Aqlane Medical

Iso Medical Devices Quality Management Systems Certification Bqai

Ofogh Sanat Medisa Company Achieved The Iso 03 Iso 9001 08 Quality Management System

Iso 03 Pocket Guide Iso 03 Specifies Requirements For A Quality Management System Where An Organization Needs To Demonstr Iso Guide Iso

En Iso 12 Archives Mobile Shower Commode Chairs Raz Design Inc

Iso 03 Xenamed Industries

Iso 03 Certification Services In Andheri West Mumbai Beuna Vista Consultancy Certification Services Id

Can Csa Iso 03 Rovers Medical Devices

Hs Design Received Bsi Recertification For Operating Qms That Complies With Iso 9001 08 Iso 03 Hs Design

Portland Facility Passes Iso Certification Audit Milwaukee Electronics Companies Blog

Iso 16 New Revision Of Qms For Medical Devices Iso Consulting Services

Iso 03

Study Iso Pdf Quality Management System Audit

Sustainability Iso 03 Mind Map

Registrar Of Iso 03 Certificate In Bălcești Romania Iso 03 In Săliștea De Sus Romania Iso 03 Certification In Fierbinți Targ Romania Iso 03 Certification Registrar In Cavnic Romania Certification Service Of Iso

Iso 03 Abnova Has Extended The Scope Of Iso 03 News About Us Abnova

Certificate Iso 03 Canada Hettich

Iso

Kerecis Limited Receives Iso 03 Quality Management System Certification Kerecis
Differences Between Iso And Iso 9001

Iso 03 Iso Certifications lto Scientific

Iso 03 Certificate 15 Philips Innovation Services

Amazon In Buy Complete Iso 03 Quality Management System Book Online At Low Prices In India Complete Iso 03 Quality Management System Reviews Ratings

Services Iso 03 Cmdms Certification In Offered By Pic Certification Private Limited Id

Iso 16 Certification Qc Certification

Litron Inc Receives Iso 03 Certification

Iso 03 Medical Devices Qms Certification In Chennai noor Global

Iso 03 Ppunch Precision Punch And Tooling

Medsource Dual Seals Individually Wrapped Sterile Hydrogel Iso 03 Pk 2 487j18 Ms Hhdsk01 Grainger

Iso 03 Certification Services In Sector 16 Noida Qfs Management System Llp Id
Fda 21 Cfr Part 0 Vs Iso 16 Vs Iso 03

Interpretacao Iso 9001 08 Iso 03 E Rdc 16 13 Free Download Borrow And Streaming Internet Archive

Iso 03 Translated Into Plain English

Zertifikat Iso 03 Ac 09 Heraeus Kulzer

Iso 03
Iso 16 New Terms To Take Note Of Qmswrapper

Tanis Receives Iso 03 Certification News And Press Releases

Iso 03 Iso Quality Management

Iso 03 Quality Management System For Medical Device Certification Consulting Buy Iso Consulting Iso Consulting Medical Certification Product On Alibaba Com

Airinspace Sup Sup Compliant With Iso 03 Standard Airinspace

Iso 03 Leader Life Camara Hiperbarica Medicina Hiperbarica

Hui Awarded Medical Device Iso 03 Certification
Iso 16 Revisions Webinar

Gowanda Electronics Achieves Iso 03 Certification Gowanda Electronics

En Iso 03 Ac 07 Medical Devices Quality Management Systems Requirements For Regulatory Purposes Iso 03

Iso Iqs International
Internal Quality Management System Audit Checklist Iso 03 Iso 9001 00 Q

Pdf Iso 03 Implementation Reference Model From The Malaysian Smes Medical Device Industry

Iso 03 Banners Standard Flags

Press Release Empirical Bioscience Upgrades To Iso 16 Certification Empirical Bioscience
Iso 16 Revisions Webinar

Iso 16 Vs Iso 03

Onkocet

Iso Mdsap

Differences Iso 12 And Iso 15

En Iso 03 Ac 09 Compware Medical Gmbh

Vexos Shenzhen China Facility Achieves Iso 03 Registration Of Its Quality Management System

Iso 03 And Fda Qsr 21 Cfr 0 Internal Audit And Gap Analysis Checklist Isoxpress Amazon Com Books

Softneta Is Certified By Iso Standard Iso 03 Softneta

Spellman S China Facility Receives New Iso Certifications

Sarsen Technology Blog Dave Embedded Systems Now Iso Certified For Medical Applications

Iso 16 Six Key Differences For Medical Device Companies

Csoft Earns Iso 03 Certification For Medical Device Industry Csoft International

Softneta Is Certified By Iso Standard Iso 03 Softneta

Iso 03 Certification Services In New Delhi Delhi

Iso 03 Certification Service In Kalighat Kolkata Q Matrix Consultancy Services

Delsys We Are Now An Iso 03 Facility Certifying That We Will Consistently Provide Products That Meet Customer And Regulatory Requirements T Co Z0ufq7eznb

Onkocet

Jcs Acquistive Infotech

Iso 03 Abnova Has Just Renewed Its Iso 03 Certification News About Us Abnova

Bs En Iso Medical Devices Quality Management Systems Requirements For Regulatory Purposes

Transition Of Iso 03 To 16 Rx 360

Iso 03 Certification Services In Panchkula Fqc Certification Private Limited Id

Iso 03 Leader Life Camara Hiperbarica Medicina Hiperbarica
.jpg)
Ansi mi Iso 03 R09 Medical Devices Quality Management Systems Requirements For Regulatory Purposes



