Novozym 435
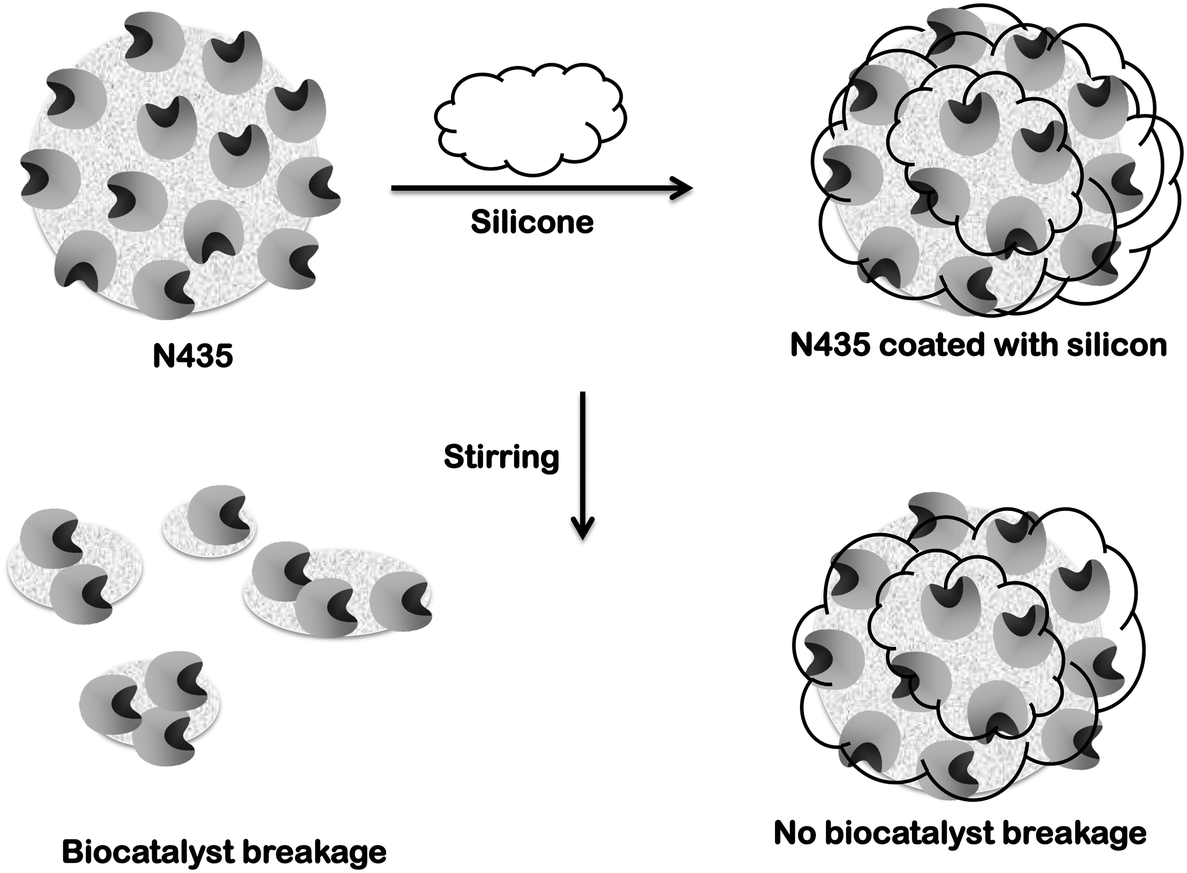
The inside cover picture shows a scanning electron microscope image of a particle of the biocatalyst Novozym 435, a lipase immobilized on a macroporous carrier A silicone coating can aid the mechanical and leaching stability of catalyst while retaining its activity, as demonstrated by Thum, Ansorge‐Schumacher et al in their paper on page 455 ff.
Novozym 435. Here, we focused on a simple enzymatic epoxidation of alkenes using lipase and phenylacetic acid The immobilised Candida antarctica lipase B, Novozym 435 was used to catalyse the formation of peroxy acid instantly from hydrogen peroxide (H 2 O 2 ) and phenylacetic acid The peroxy phenylacetic acid generated was then utilised directly for in situ oxidation of alkenes. Novozym 435, a commercial lipase from Candida antarctica, recombinant, expressed in Aspergillus niger, immobilized on macroporous acrylic resin, has been already described in the obtention of biodiesel It is here evaluated in the production of a new biofuel that integrates the glycerol as monoglyceride (MG) together with two fatty acid ethyl esters (FAEE) molecules by the application of 1,3selective lipases in the ethanolysis reaction of sunflower oil. In this study, deep eutectic solvent (DES) used as co solvent for enzymatic biodiesel production from degumming palm oil (DPO) DES is formed from the salt compound cholinechloride (ChCI) with glycerol at 12 molar ratio Furthermore, the effectiveness of the DES was tested by enzymatic reactions using novozym 435® for the production of palm biodiesel with raw materials DPO.
The effects of the reaction medium and substrate concentration were studied on the selectivity of Novozym 435 using the asymmetric hydrolysis of dimethyl3phenylglutarate as a model reaction Results show that the use of choline chloride ChClurea/phosphate buffer 50% ( v/v ) as a reaction medium increased the selectivity of Novozym 435 by 16% (ee = %) with respect to the one in 100% phosphate buffer (ee = 76%). Strem Chemicals, Inc 7 Mulliken Way Newburyport, MA USA Tel (978) 499 1600 Fax (978) 465 3104 Toll free (in USA & Canada) Tel (800) 647 8736. TETRAHEDRON LETTERS Pergamon Tetrahedron Letters 44 (03) 8453–8455 Enhanced selectivity in Novozym 435 catalyzed kinetic resolution of secondary alcohols and butanoates caused by the (R)alcohols Elisabeth Egholm Jacobsen, Erik van Hellemond, Anders Riise Moen, Lucia Camino Vazquez Prado and Thorleif Anthonsen* Department of Chemistry, Norwegian University of Science and Technology, N7491 Trondheim, Norway Received 28 July 03;.
Novozym 435 Introduction Human milk is the ideal source of nutrients for newborn infants, which could supply energy and essential nutrients to infants1 Furthermore, human milk fat (HMF) represents the primary source of energy for the breastfed baby and it provides 50–60% dietary energy of the infants’ required dietary2. Results were compared with those obtained under the same conditions using a traditional, but more expensive, commercial biocatalyst, Novozym 435 (lipase B from C antarctica immobilized on Lewatit VP OC) When NS 011 was used in the polymerization of globalide, longer reaction times (240 min)—when compared to Novozym 435—were required to. Mixtures of 1(3)monostearin and distearin were prepared by direct esterification of glycerol with stearic acid or transesterification using ethyl stearate as acyl donor in the presence of Candida antarctica lipase (Novozym 435) using a variety of solvents of differing polarity In all cases, the transesterification resulted in higher product yields.
Commercial lipase from Candida antarctica (Novozym 435), immobilized on a macroporous anionic resin (012U/g, 14% water, diameter in the range of 0309mm and optimum temperature of 70ºC), was purchased from Novozymes (Araucária, PR, Brazil) and used as catalyst in all tested systems. Stabilité fonctionnelle de la Novozyme® 435 au cours de la synthèse enzymatique du myristate de mannosyle en liquide ionique pur Lors de l’élaboration d’une voie de synthèse biocatalysée, la réutilisation de l’enzyme est un paramètre important à considérer pour la réduction des couts industriels. View 0 peer reviews of Enhanced selectivity in Novozym 435 catalyzed kinetic resolution of secondary alcohols and butanoates caused by the (R)alcohols on Publons COVID19 add an open review or score for a COVID19 paper now to ensure the latest research gets the extra scrutiny it needs.
Novozym 435catalyzed deacetylation in the presence of excess nbutanol in THF 35 After column chromatography, the target compounds 4, 6, 8, 12, 14 and 16 were obtained in good yields A high chemoselectivity was observed for deacylation of compounds 3, 7, 11 and 15, the aromatic acid ester. Novozymes novozym 435 Novozym 435, supplied by Novozymes, used in various techniques Bioz Stars score 90/100, based on 114 PubMed citations ZERO BIAS scores, article reviews, protocol conditions and more. Mixtures of 1(3)monostearin and distearin were prepared by direct esterification of glycerol with stearic acid or transesterification using ethyl stearate as acyl donor in the presence of Candida antarctica lipase (Novozym 435) using a variety of solvents of differing polarity In all cases, the transesterification resulted in higher product yields.
The effects of the pretreatment of immobilized Candida antarctica lipase enzyme (Novozym 435) on methanolysis for biodiesel fuel production were investigated Methanolysis progressed much faster when Novozym 435 was preincubated in methyl oleate for 05 h and subsequently in soybean oil for 12 h. Novozym 435 (N435) is a commercially available immobilized lipase produced by Novozymes It is based on immobilization via interfacial activation of lipase B from Candida antarctica on a resin, Lewatit VP OC 1600 This resin is a macroporous support formed by poly (methyl methacrylate) crosslinked with divinylbenzene. Novozym® 435 is a CALB lipase immobilized on a hydrophobic carrier (acrylic resin).
Found that Novozym ® 435 was more active in esterification of levulinic acid with nbutanol As our results suggested, the nonspecific lipase Novozym ® 435 exhibited less steric hindrance for shorter chain organic acids as compared to other lipases, and showed higher activity in direct esterification. Novozym 435 was packed in a packedbed reactor and used to catalyze the alcoholysis of methanol and soybean oil to produce FAMEs in a cosolvent system. Heterogeneous biocatalysis is a part of biotechnology and it has commercial potential for industrial implementation, in particular the final stages of deep processing of renewable raw materials The commercially attractive heterogeneous biocatalysts are prepared by immobilizing practically valuable enzymatic active substances onto solid inorganic supports.
Novozym 435 (N435) is a commercially available immobilized lipase produced by Novozymes It is based on the immobilization via interfacial activation of the lipase B from Candida antarctica on a. Novozyme 435 (immobilized on acrylic resin) EMAIL THIS PAGE TO A FRIEND To Email From Email Message SigmaAldrich Novozyme 435 (immobilized on acrylic resin) MDL number MFCD SDS Certificate of Analysis (COA) Purchase;. The 2PEAc was synthesized using Novozym ® 435 in a packedbed reactor with a so lventfree system, the reaction para meters affecting the synthesis of 2PEAc were evaluated, and the response surface methodology (RSM) using a threelevelthreefactor Box Behnken design was conducted to determine the optimal condition of.
Razvoj novih inkapsulacionih i enzimskih tehnologija za proizvodnju biokatalizatora i biološki aktivnih komponenata hrane u cilju povećanja njene konkurentnosti, kvaliteta i bezbednosti (MPNTR ). Novozym‐435‐catalysed esterification of caprylic acid, capric acid and oleic acid with glycerol for the synthesis of medium‐ and long‐chain triglycerides (MLCT) in vacuum and solvent‐free system was investigated in this study Response surface methodology with a three‐level, four‐factorial design was applied to optimise the. The coating of the immobilized lipase Novozyme 435 (NZ435), as a model enzyme preparation, with different silicone loadings was studied in detail by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), as well as by energy‐dispersive X‐ray spectroscopy (EDX) and BET isotherms, and offers explanations and prerequisites for its stabilizing effects.
Novozym 435 acts fast and its optimal specific activity (g BD/h/g catalyst) is 50fold higher than that of Amberlyst 15 The maximum BD yields obtained using Novozym 435 and Amberlyst 15 are 95 and 97%, respectively Both catalysts are recycled more than 15 cycles without losing their activities. Novozym® 435 is a CALB lipase immobilized on a hydrophobic carrier (acrylic resin). Accepted 12 September 03 Abstract—In esterifications.
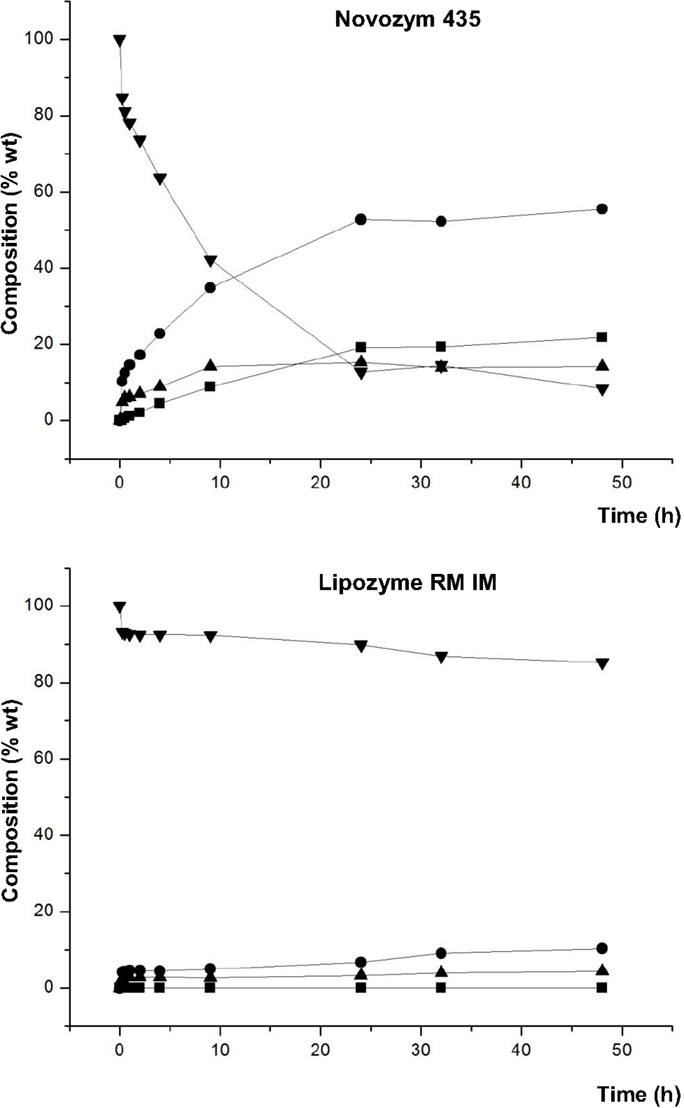
DES is formed from the salt compound cholinechloride (ChCI) with glycerol at 12 molar ratio Furthermore, the effectiveness of the DES was tested by enzymatic reactions using novozym 435®for the production of palm biodiesel with raw materials DPO. Novozym® 435 proves to be a more efficient biocatalyst than Lipozyme® RMIM Wax esters are longchain esters that have been widely applied in premium lubricants, parting agents, antifoaming agents and cosmetics. Biodiesel has been receiving significant attention as a renewable and nonpolluting fuel In this study, oleic acid and bioalcohols (ethanol and butanol) were used as substrates for biodiesel production The reactions were performed in a solventfree system using immobilized lipase (Novozym 435) as biocatalyst in a batch esterification process.
Several studies have reported that immobilized Candida antarctica lipase (Novozym 435), can effectively catalyse the methanolysis of soya‐bean oils without extra solvents and most of them focused only on refined soya‐bean oil sources 8 – 13. In this work the Novozym® 435, N435, has been employed The catalyst is based on the Candida antartica lipase B with immobilization on an acrylic macroporous resin N435 has been investigated for biodiesel production from both mixtures of oils and FFAs, and from pure oils,,. Enzymatic synthesis of welldefined sophorolipid analogues for evaluation of their bioactivities and as new building blocks for the preparation of glycolipidbased amphiphilic polymers is described Lipase Novozym 435 from Candida antarctica has been shown to be an efficient catalyst for acylation of sophorolipids esters A mixture of sophorolipids produced by Torulopsis bombicola was.
Yasutaka Shimotori, Tetsuo Miyakoshi, Combination of Novozym 435–Catalyzed Hydrolysis and Mitsunobu Reaction for Production of ( R )γLactones , Synthetic Communications, /, 40, 11, (), (10). Novozym‐435‐catalysed esterification of caprylic acid, capric acid and oleic acid with glycerol for the synthesis of medium‐ and long‐chain triglycerides (MLCT) in vacuum and solvent‐free system was investigated in this study. Commercially available lipases, such as Novozym 435 (immobilized lipase B from Candida antarctica) and Lipozyme IM (immobilized lipase from Rhizomucor miehei), also efficiently catalyze esterification reactions between long chain fatty acids and fatty alcohols 44, 45 After isolation and further characterization, the jojoba lipases can be.
IR contact We encourage all shareholders and investment professionals to contact us, should you have any questions or requests concerning Novozymes. The reaction mixture contained 500 µl of microbial lipids, 12 mg of Novozym 435 and 125 µl of methanol and was incubated at 37 °C in an orbital shaker at 160 rpm Four addition each of 125 µl of methanol were done every 24 h and the reaction was stopped after 168 h by adding 25 ml of n hexane. Feasible Novozym 435Catalyzed Process to Fatty Acid Methyl Ester Production from Waste Frying Oil Role of Lipase Inhibition Laura Azócar, Gustavo Ciudad, Robinson Muñoz, David Jeison, Claudio Toro and Rodrigo Navia Scientific and Technological Bioresource s Nucleous, La Frontera University Chile 1.
Esterification using Novozym 435 did not lead to DAG formation which was confirmed by injecting standard reference standard of glyceryl 1,3distearate on HPLC Based on these results 2h reaction time was found to be the most optimum time for esterification using Novozym 435 as catalyst. Novozym435 has been found to be an effective biocatalyst for the kinetic resolution of a series of racemic 2,3allenols, affording highly optically active (S)()2,3allenols and (R)()2,3allenyl acetates in high yields and with excellent ee values. The effects of the pretreatment of immobilized Candida antarctica lipase enzyme (Novozym 435) on methanolysis for biodiesel fuel production were investigated Methanolysis progressed much faster when Novozym 435 was preincubated in methyl oleate for 05 h and subsequently in soybean oil for 12 h.
Date HS Code Description Origin Country Port of Discharge Unit Quantity Value (INR) Per Unit (INR) Nov 21 16 NOVOZYM INDUSTRIAL ENZYME Denmark. The selectivity of Novozym 435, an immobilized Candida antarctica lipase B toward linoleic (LA), conjugated linoleic (CLA), and pinolenic acids (PLA) was investigated in the esterification of glycerol with an equimolar (273 mol% each) mixture of the fatty acids (FAs) in a solventfree system to prepare triacylglycerols (TAGs) with antiobesity effects. Figure Legend Snippet Lipasecatalyzed acylation of galactosides 30 mM of aromatic galactosides were reacted with an equivalent amount of donor substrates with Novozym 435 ( mg/mL) and 100 mg/mL of molecular sieves (5Å, 8–12 mesh) in 2methyl2propanol The enzymatic reaction was carried out for 24 h at 75°C with constant stirring.
Novozym 435‐catalyzed esterification of glycerol with an equimolar mixture of linoleic, conjugated linoleic, and pinolenic acids. Enzyme and microbial technology, 84, undefined (1622) The first Novozym 435 lipasecatalyzed MoritaBaylisHillman (MBH) reaction with amides as cocatalyst was realized Results showed that neither Novozym 435 nor amide can independently catalyze the reaction This cocatalytic system that used a catal. Such as Novozym 435, Lipozyme TL IM and Lipozyme RM IM 12–14The advantage of enzymatic interesterification is that it can perform under a mild condition, which effectively prevents the migration of fatty acids and production of trans fatty acids, decreases the consumption of energy.

The Reaction Scheme Of Novozym 435 Catalyzed Cqa Acylation With Download Scientific Diagram
2

Novozym 435 Price Bioz Ratings For Life Science Research
Novozym 435 のギャラリー
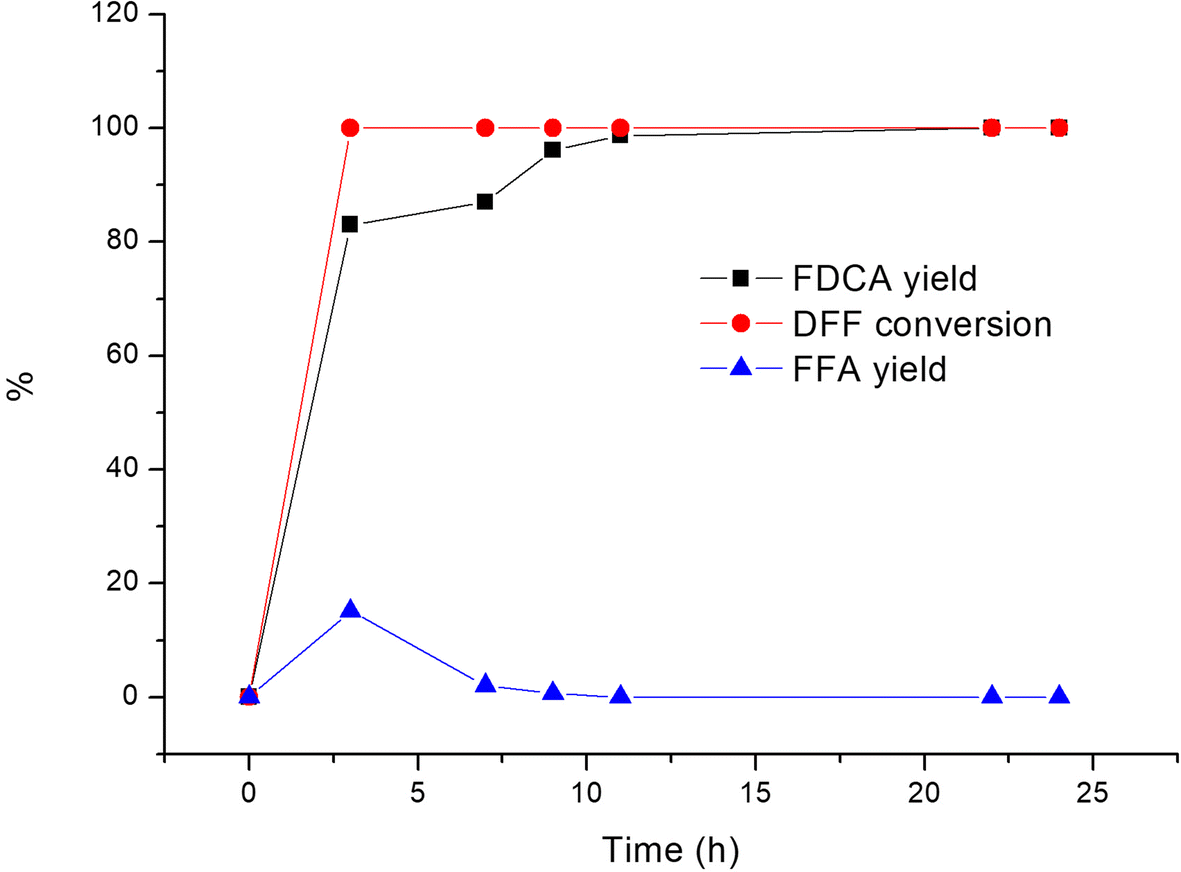
Figure 3 A Novel 2 5 Furandicarboxylic Acid Biosynthesis Route From Biomass Derived 5 Hydroxymethylfurfural Based On The Consecutive Enzyme Reactions Springerlink

Stability To Low Temperatures Of Novozym 435 A And Lipozyme Im B Download Scientific Diagram

Estolides Synthesis Catalyzed By Immobilized Lipases

Novozym 435 Displays Very Different Selectivity Compared To Lipase From Candida Antarctica B Adsorbed On Other Hydrophobic Supports Sciencedirect

Novozym 435 Price Bioz Ratings For Life Science Research
Www Novozymes Com Media Project Novozymes Website Website Document Library Advance Your Business Pharma Biocatalysis Brochure Immobilised Lipases Pdf

Figure 1 Effect Of Alcohol Structure On The Optimum Condition For Novozym 435 Catalyzed Synthesis Of Adipate Esters
Http Ir Nsfc Gov Cn Paperdownload Pdf
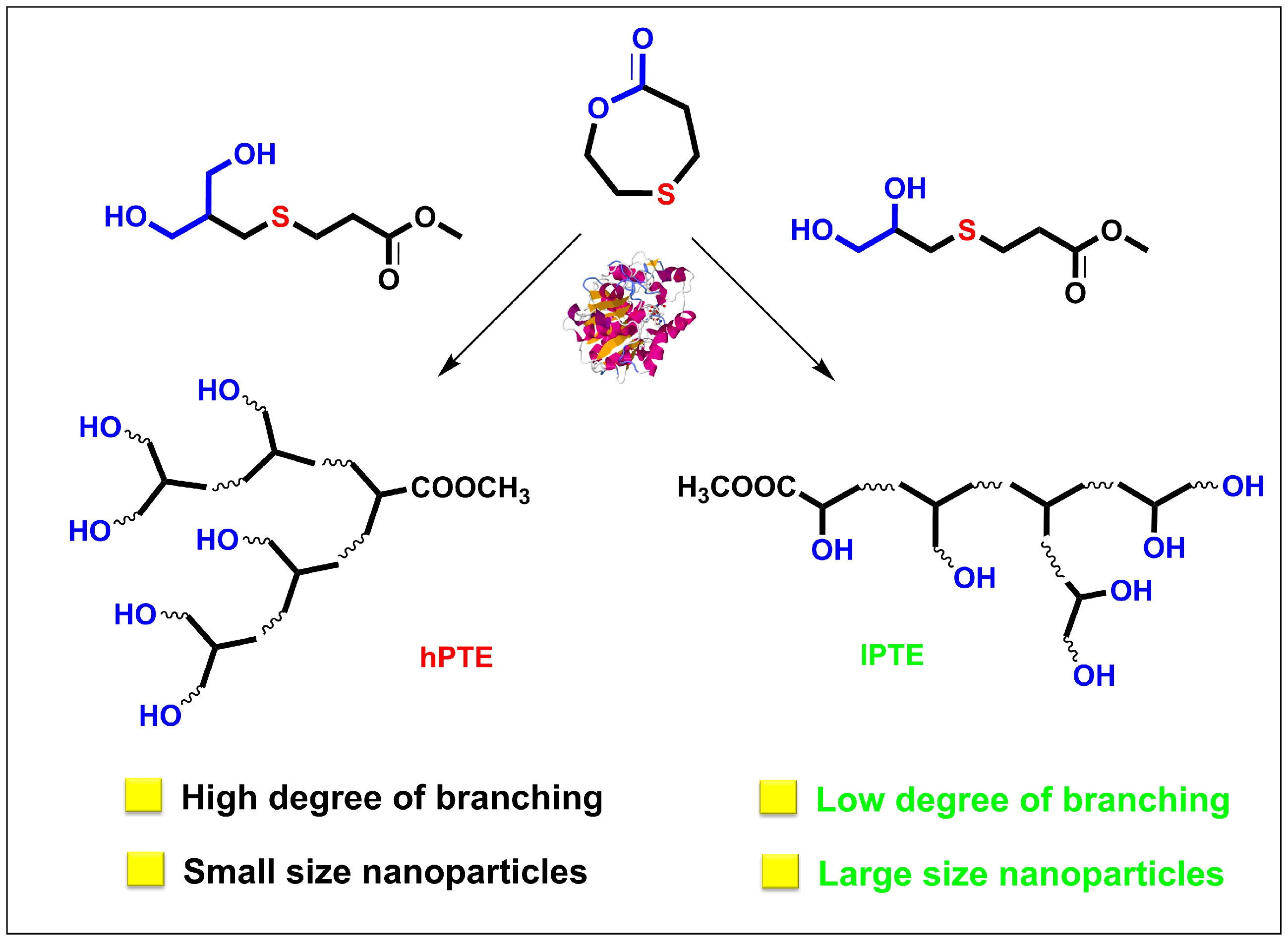
Molecules Free Full Text Novozym 435 Catalyzed Synthesis Of Well Defined Hyperbranched Aliphatic Poly B Thioether Ester
Plos One Rutin Derivatives Obtained By Transesterification Reactions Catalyzed By Novozym 435 Antioxidant Properties And Absence Of Toxicity In Mammalian Cells

Enzymatic Biodiesel Synthesis Using A Byproduct Obtained From Palm Oil Refining

Successive Cycles Of Utilization Of Novozym 435 In Three Different Reaction Systems
Www Degruyter Com Downloadpdf Journals Boca 3 1 Article P27 Xml
Www Novozymes Com Media Project Novozymes Website Website Document Library Advance Your Business Pharma Biocatalysis Brochure Immobilised Lipases Pdf

Biocatalysis In Organic Chemistry Ntnu

Enzymatic Synthesis Of Functional Structured Lipids From Glycerol And Naturally Phenolic Antioxidants Intechopen
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg
Rational Enhancement Of Enzyme Catalyzed Enantioselective Reaction By Construction Of Recombinant Enzymes Based On Additive Strategy Springerlink

Enzymatic Kinetic Resolution With Novozyme 435 Download Table

Production Of A Biodiesel Like Biofuel Without Glycerol Generation By Using Novozym 435 An Immobilized Candida Antarctica Lipase Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For
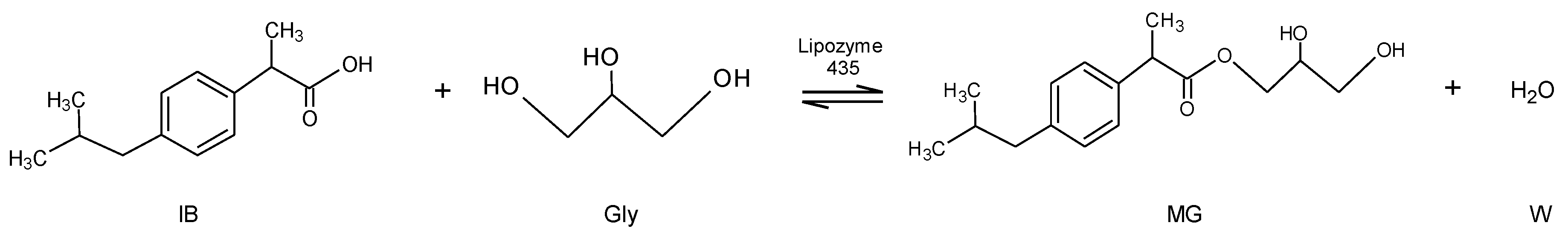
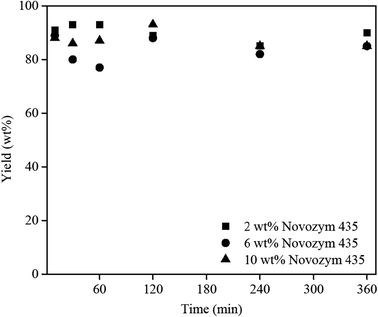
Catalysts Free Full Text Synthesis Of Ibuprofen Monoglyceride In Solventless Medium With Novozym 435 Kinetic Analysis Html

Novozym 435 Sigma Aldrich Bioz Ratings For Life Science Research

Substrate Selectivity Of Novozym 435 In The Esterification Of Glycerol With An Equimolar Mixture Of Linoleic Conjugated Linoleic And Pinolenic Acids Woo 16 European Journal Of Lipid Science And Technology Wiley Online Library
Polyesters From Macrolactones Using Commercial Lipase Ns 011 And Novozym 435 As Biocatalysts Springerlink

First Novozym 435 Lipase Catalyzed Morita Baylis Hillman Reaction In The Presence Of Amides Semantic Scholar

The Control Of Novozym 435 Chemoselectivity And Specificity By The Solvents In Acylation Reactions Of Amino Alcohols Sciencedirect
Www Mdpi Com 14 3049 24 4 792 Pdf Vor

Pdf Novozym 435 The Perfect Lipase Immobilized Biocatalyst Semantic Scholar
Frontiers Solvent Free Lipase Catalyzed Synthesis Of Diacylgycerols As Low Calorie Food Ingredients Bioengineering And Biotechnology
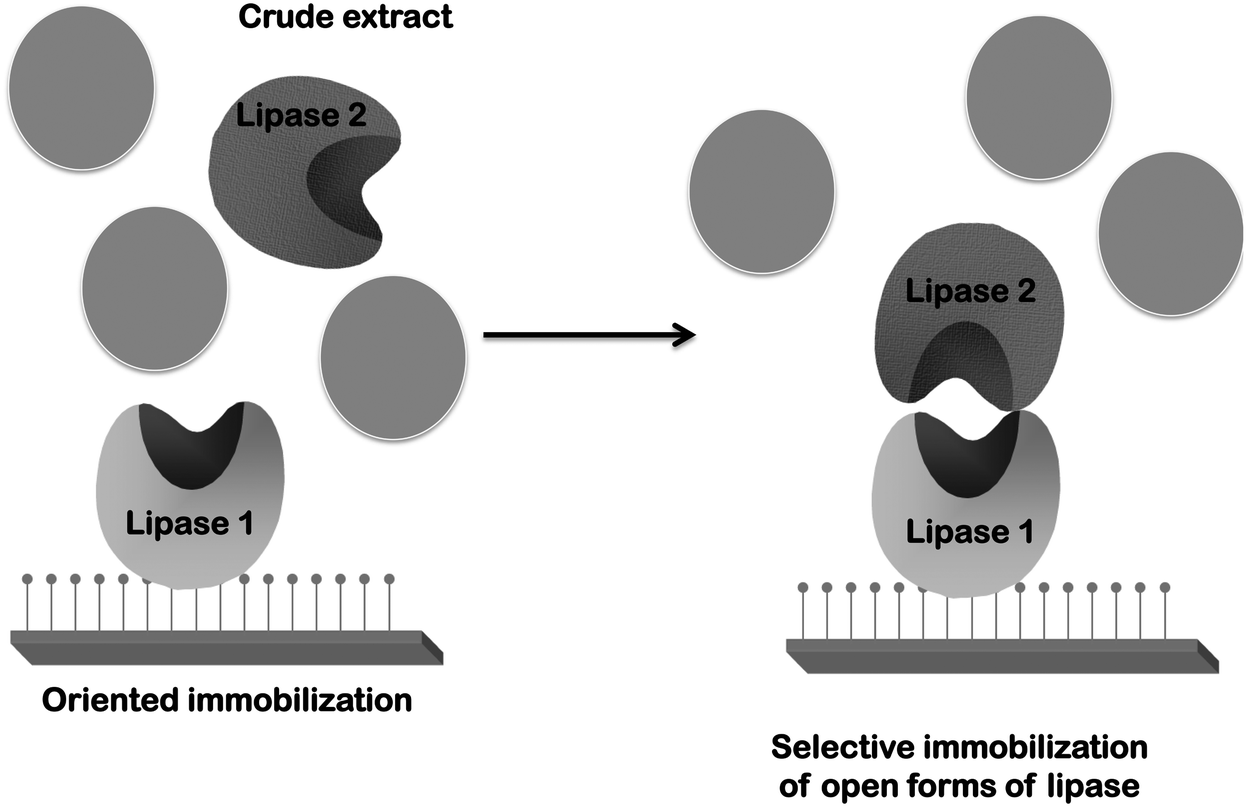
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg

Strem Chemical Inc Cas 9001 62 1 5g Novozym 435 Novozym 435 5g Fisher Scientific

Immobilized Lipase Novozyme 435 Cas 9001 62 1 Haihang Industry
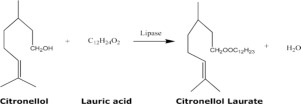
Ultrasound Assisted Synthesis Of Citronellol Laurate By Using Novozym 435 Catalysis Today X Mol

Tlc Butanol 4 Water 0 25 Ethanol 0 25 And Acetic Acid 0 5 Of The Transesterification Reaction Of The Flavonoid Rutin With The Acyl Donor Vinyl Acetate Catalyzed With The Enzyme Novozym 435 And The Respective Products
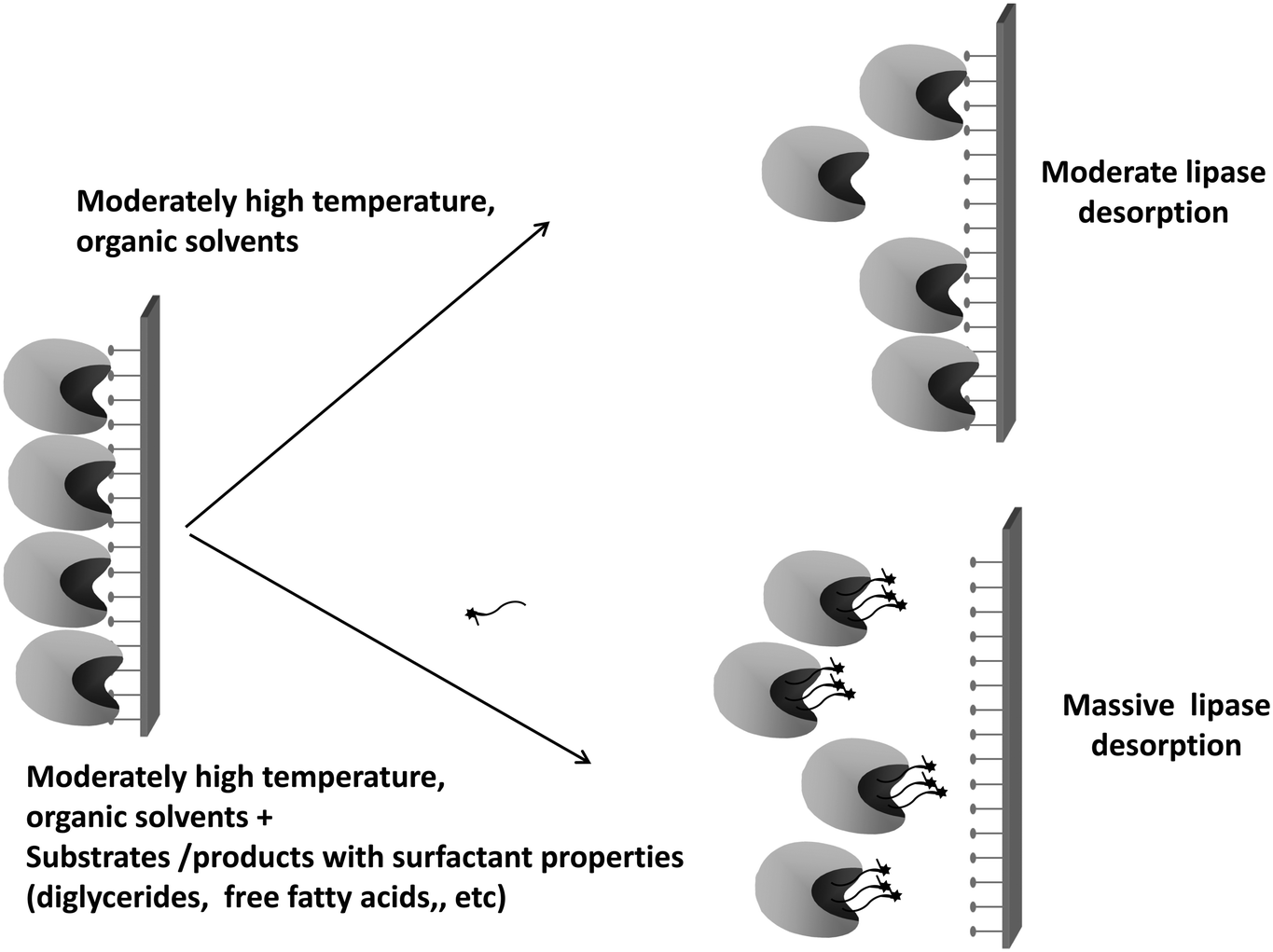
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg

Pdf Combination Of Novozym 435 Catalyzed Enantioselective Hydrolysis And Amidation For The Preparation Of Optically Active D Hexadecalactone Semantic Scholar

Novozym 435 Mediated Epoxidation Of Alkenes Download Table
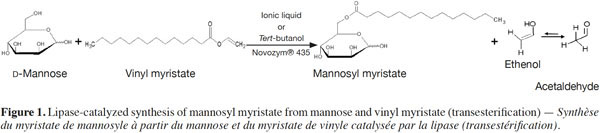
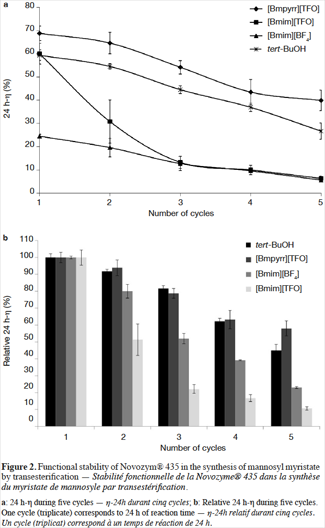
Reusability Study Of Novozym 435 For The Enzymatic Synthesis Of Mannosyl Myristate In Pure Ionic Liquids Universite De Liege

Lipase Catalyzed Synthesis And Characterization Of 6 O 11 Dodecenoic Glucose Ester In Ionic Liquids

The Rise Of Biocatalysis In Continuous Flow Syrris Chemistry Blog

Novozym 435 Catalyzed Synthesis Of Fructose Mono And Diesters Download Scientific Diagram
Development Of Zero Trans Shortenings With High Thermo Oxidative Stability By Enzymatic Transesterification

Novozym 435 Price Bioz Ratings For Life Science Research

Woa1 A Process For Preparing The Key Intermediate Of Apremilast Using Enzymatic Resolution Of The Racemic Amines Google Patents

Insight Into Microwave Assisted Immobilized Candida Antarctica Lipase B Catalyzed Kinetic Resolution Of Rs Ketorolac Sciencedirect

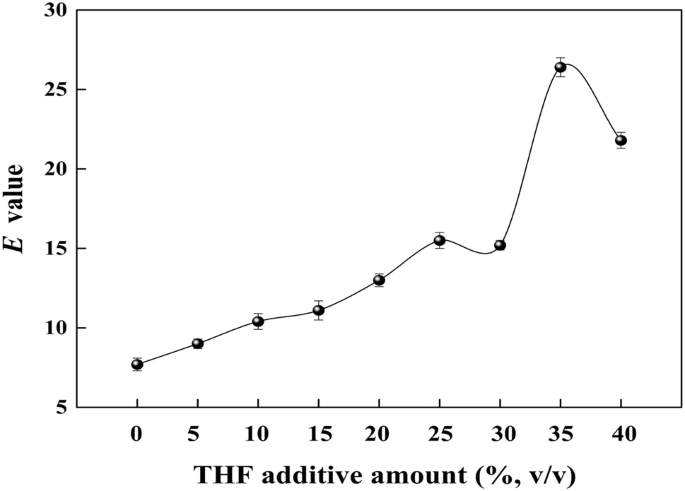
Increased Selectivity Of Novozym 435 In The Asymmetric Hydrolysis Of A Substrate With High Hydrophobicity Through The Use Of Deep Eutectic Solvents And High Substrate Concentrations Abstract Europe Pmc
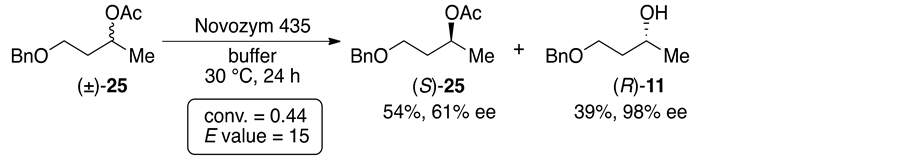
Enzyme Mediated Enantioselective Hydrolysis Of Aliphatic Dicarboxylic Acid Diesters
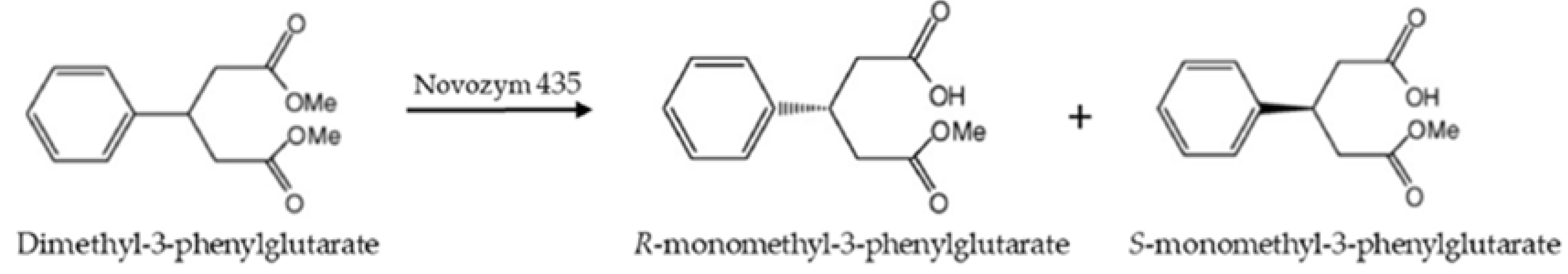
Molecules Free Full Text Increased Selectivity Of Novozym 435 In The Asymmetric Hydrolysis Of A Substrate With High Hydrophobicity Through The Use Of Deep Eutectic Solvents And High Substrate Concentrations Html
Http Pubs Acs Org Doi Pdf 10 1021 Acs Iecr 6b
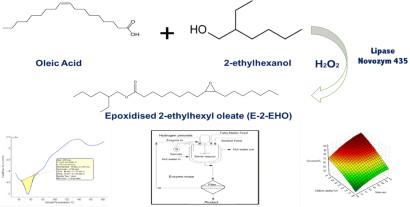
A Cleaner Enzymatic Approach For Producing Non Phthalate Plasticiser To Replace Toxic Based Phthalates Springerprofessional De

Figure 1 Artificial Neural Network Modeling Studies To Predict The Yield Of Enzymatic Synthesis Of Betulinic Acid Ester

Synthesis Of Various B D Glucopyranosyl And B D Xylopyranosyl Hydroxybenzoates And Evaluation Of Their Antioxidant Activities In Heterocyclic Communications Volume 23 Issue 3 17

Reusability Of Novozym 435 On Lipase Mediated Epoxidation In Scco 2 Download Scientific Diagram
Enzymatic Processes In Alternative Reaction Media A Mini Review Ghaffari Moghaddam Journal Of Biological Methods

Enzymatic Esterification Of Oleic Acid And Propanol By Novozym 435 Scientific Net
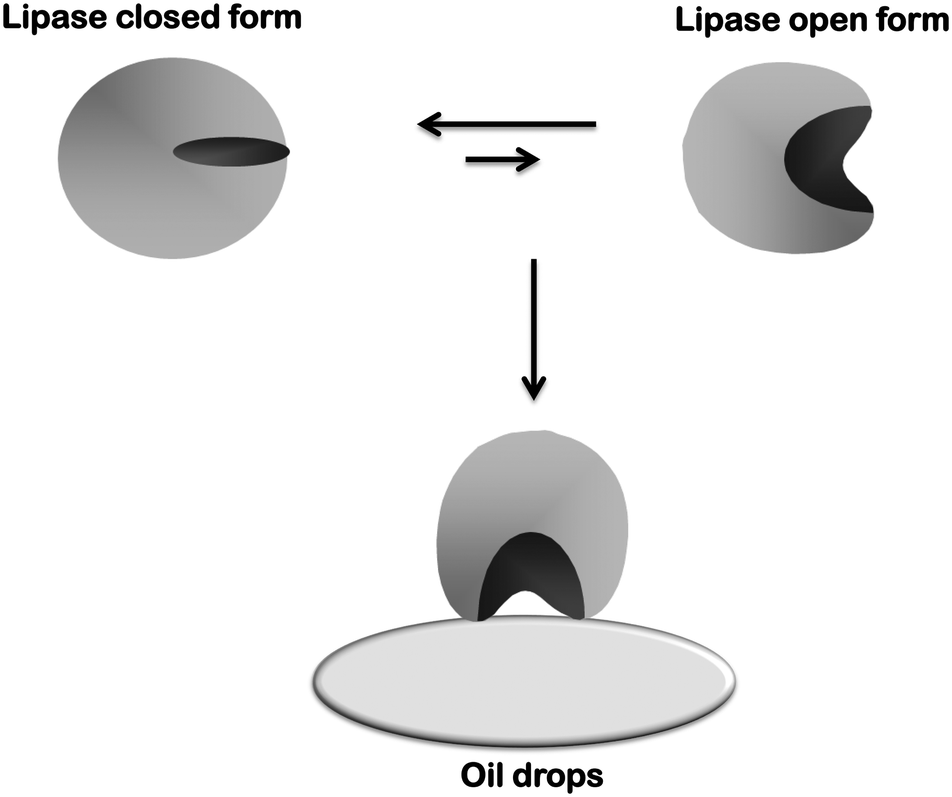
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg

Microwave Activation Of Immobilized Lipase For Transesterification Of Vegetable Oils

9 Gross

Optimization Of Solvent Free Geranyl Butanoate Production Using Novozyme 435 And Homemade Polyurethane Immobilized Novozyme Nzl 102 Lyo Hq As Catalysts

Transesterification Of Triolein And Methanol With Novozym 435 Using Co Solvents Sciencedirect
Www Novozymes Com Media Project Novozymes Website Website Document Library Advance Your Business Pharma Biocatalysis Brochure Immobilised Lipases Pdf
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg
Plos One Rutin Derivatives Obtained By Transesterification Reactions Catalyzed By Novozym 435 Antioxidant Properties And Absence Of Toxicity In Mammalian Cells
Http Pubs Acs Org Doi Pdf 10 1021 Acs Oprd 6b

Lactulose Acylation Positions With Novozym 435 And Lipozyme Tl Im Download Scientific Diagram

Laboratory Of Biocatalysis Bioprocessing Rpi

Enzymes For Biocatalysts
Reusability Study Of Novozym 435 For The Enzymatic Synthesis Of Mannosyl Myristate In Pure Ionic Liquids Universite De Liege

Novozym 435 Displays Very Different Selectivity Compared To Lipase From Candida Antarctica B Adsorbed On Other Hydrophobic Supports Sciencedirect
Http Homepages Rpi Edu Grossr Doc Biomacromolecules 08 9 463 Pdf

Novozym 435 Price Bioz Ratings For Life Science Research

Successive Cycles Of Utilization Of Novozym 435 In Three Different Reaction Systems
Http Www Asianjournalofchemistry Co In User Viewfreearticle Aspx Articleid 26 3 66

Novozym 435 Price Bioz Ratings For Life Science Research
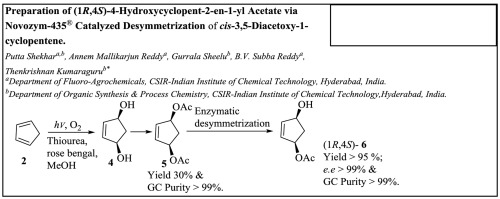
Preparation Of 1r 4s 4 Hydroxycyclopent 2 En 1 Yl Acetate Via Novozym 435 Catalyzed Desymmetrization Of Cis 3 5 Diacetoxy 1 Cyclopentene Tetrahedron X Mol
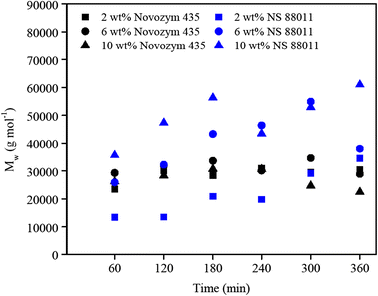
Polyesters From Macrolactones Using Commercial Lipase Ns 011 And Novozym 435 As Biocatalysts Springerlink
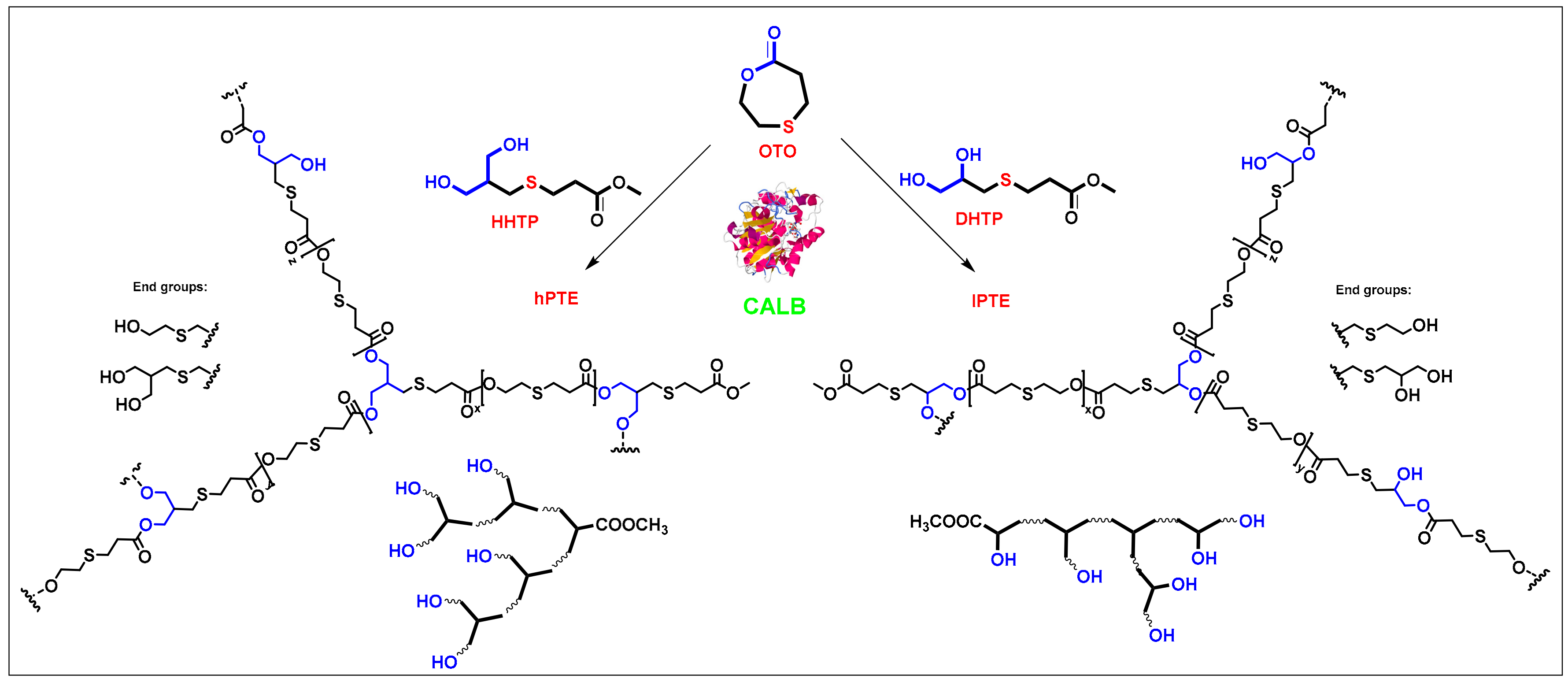
Molecules Free Full Text Novozym 435 Catalyzed Synthesis Of Well Defined Hyperbranched Aliphatic Poly B Thioether Ester

Woa1 A Process For Preparing The Key Intermediate Of Apremilast Using Enzymatic Resolution Of The Racemic Amines Google Patents

Selective Polymerization Of Functional Monomers With Novozym 435 Veld 07 Journal Of Polymer Science Part A Polymer Chemistry Wiley Online Library

Immobilized Lipase Novozyme 435 Cas 9001 62 1 Haihang Industry

Systemic Concocting Of Cross Linked Enzyme Aggregates Of Candida Antarctica Lipase B Novozyme 435 For The Biomanufacturing Of Rhamnolipids Rathankumar 19 Journal Of Surfactants And Detergents Wiley Online Library

Novozyme 435 And Lipozyme Rm Im Preferably Esterify Polyunsaturated Fatty Acids At The Sn 2 Position Rivero Pino European Journal Of Lipid Science And Technology Wiley Online Library
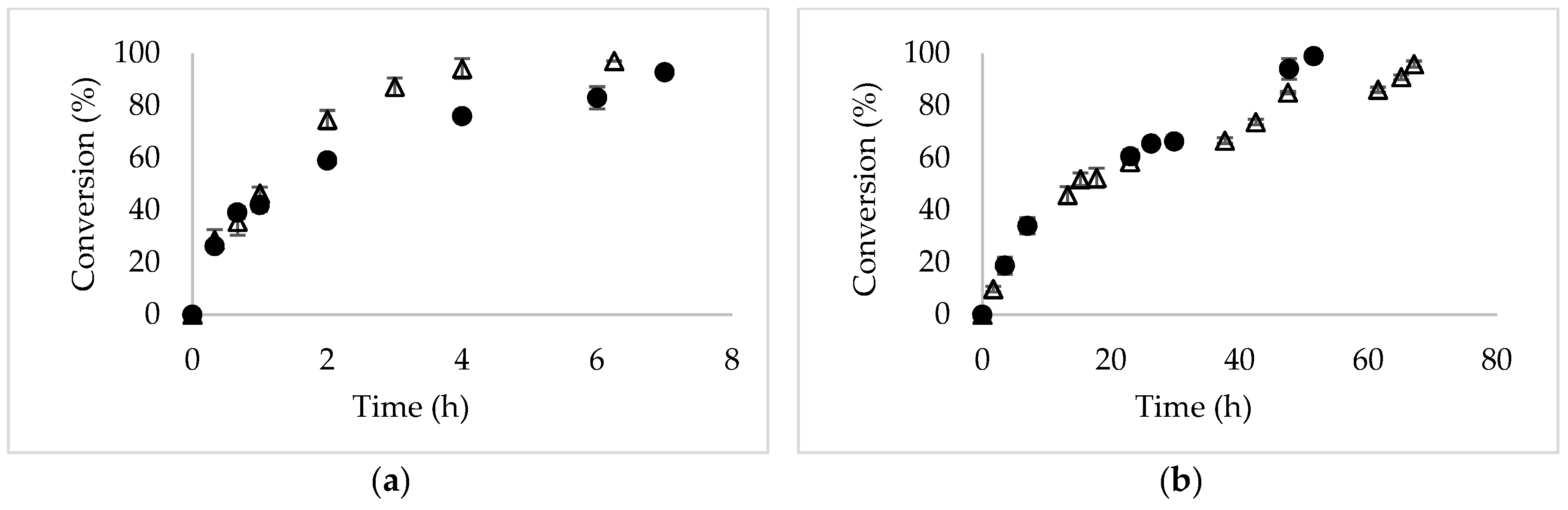
Molecules Free Full Text Increased Selectivity Of Novozym 435 In The Asymmetric Hydrolysis Of A Substrate With High Hydrophobicity Through The Use Of Deep Eutectic Solvents And High Substrate Concentrations Html
Www Novozymes Com Media Project Novozymes Website Website Document Library Advance Your Business Pharma Biocatalysis Brochure Immobilised Lipases Pdf

First Novozym 435 Lipase Catalyzed Morita Baylis Hillman Reaction In The Presence Of Amides Sciencedirect
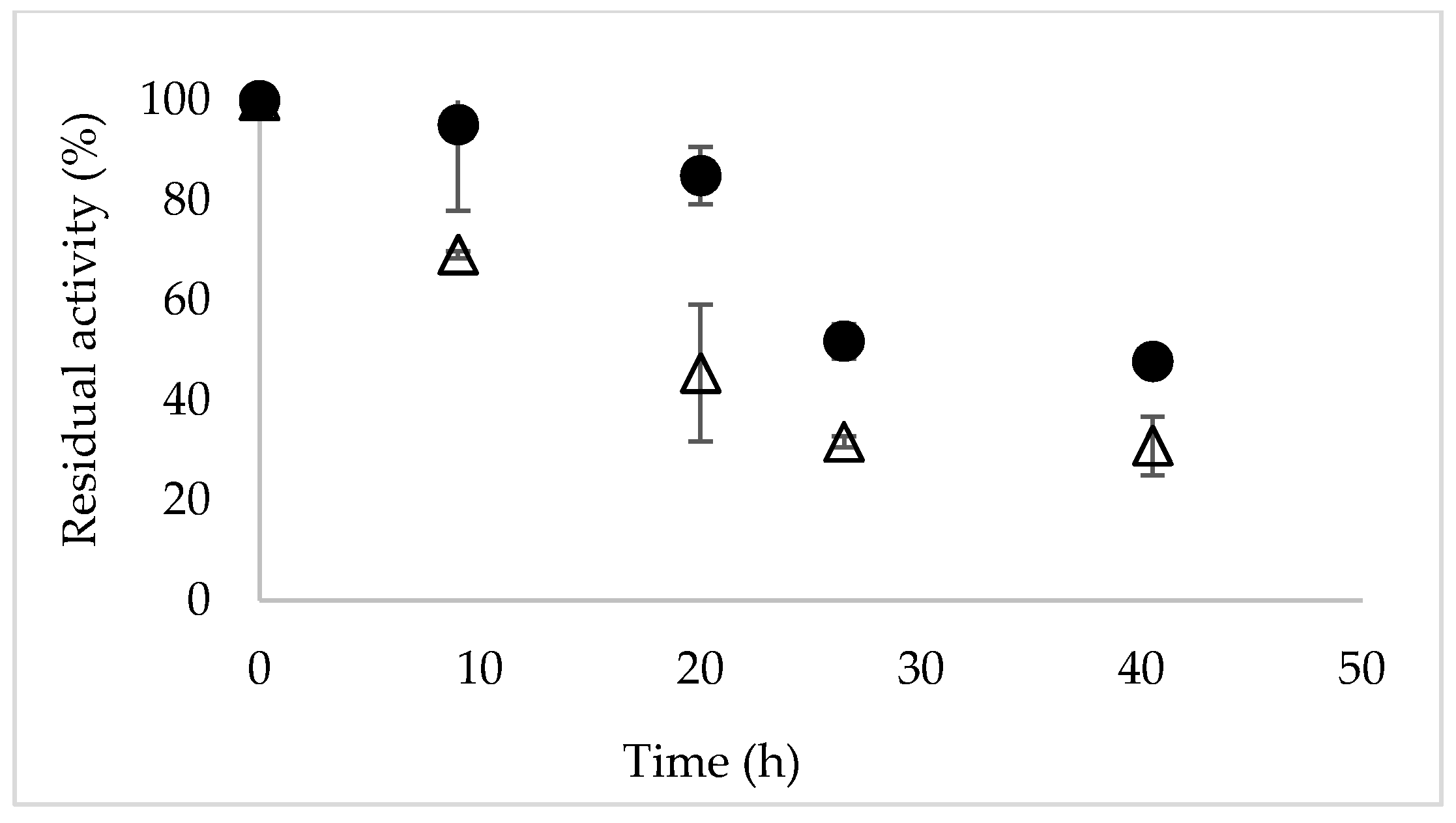
Molecules Free Full Text Increased Selectivity Of Novozym 435 In The Asymmetric Hydrolysis Of A Substrate With High Hydrophobicity Through The Use Of Deep Eutectic Solvents And High Substrate Concentrations Html

Scheme With Optimized Reaction Conditions And Enzyme Novozyme 435 As Biocatalyst By 96 H
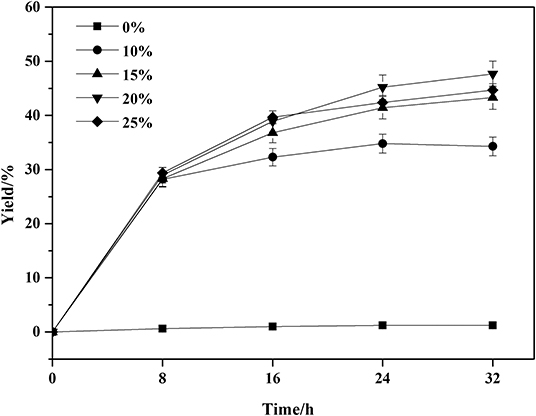
Frontiers Optimization Of The Lipase Catalyzed Selective Amidation Of Phenylglycinol Bioengineering And Biotechnology

Scheme 1 Reagents And Conditions A Novozym 435 50 60 Or 70 C Download Scientific Diagram
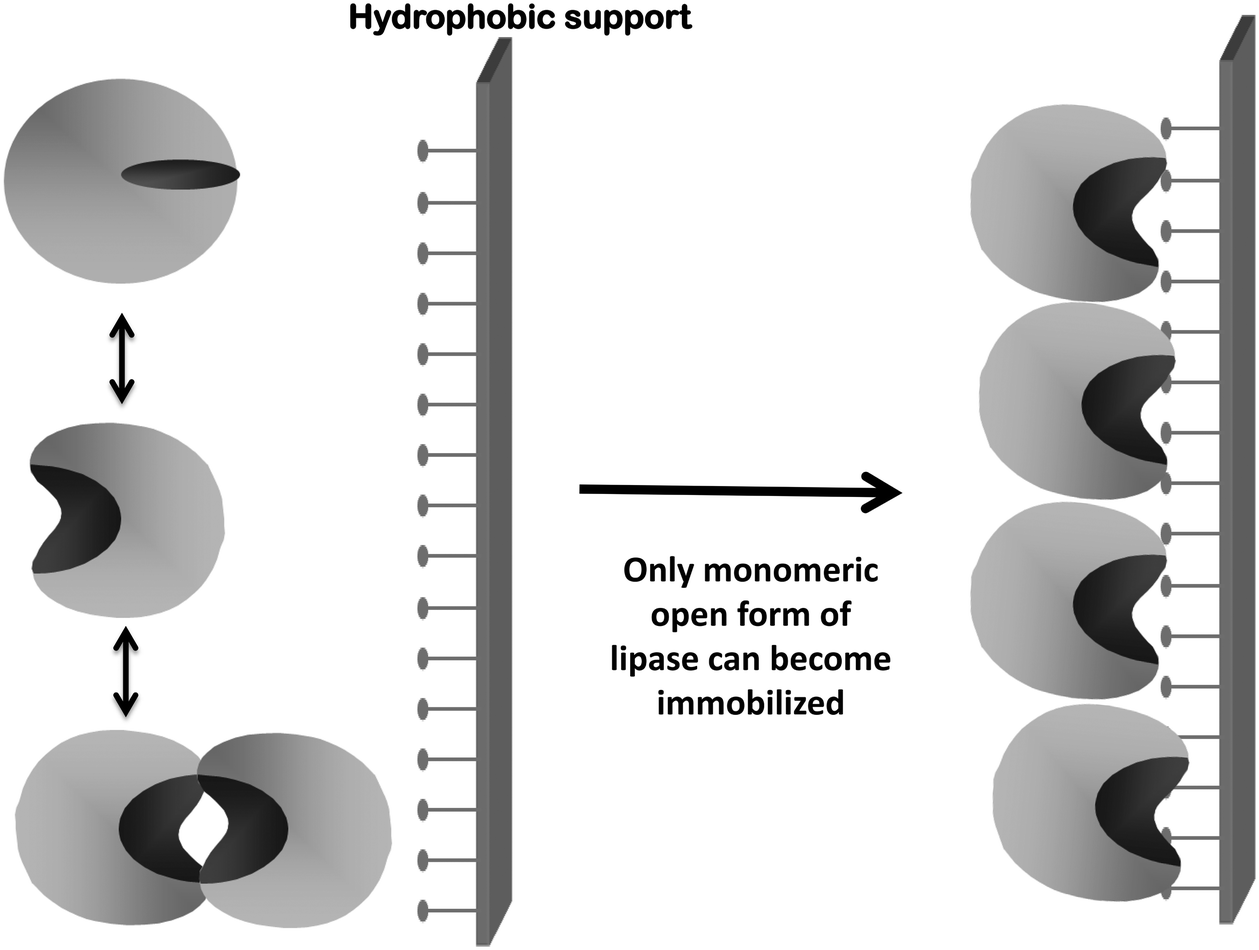
Novozym 435 The Perfect Lipase Immobilized Biocatalyst Catalysis Science Technology Rsc Publishing Doi 10 1039 C9cyg

Pdf Combination Of Novozym 435 Catalyzed Enantioselective Hydrolysis And Amidation For The Preparation Of Optically Active D Hexadecalactone Semantic Scholar
Plos One Rutin Derivatives Obtained By Transesterification Reactions Catalyzed By Novozym 435 Antioxidant Properties And Absence Of Toxicity In Mammalian Cells

Artificial Neural Network Modeling Of Biolubricant Production Using Novozym 435 And Castor Oil Substrate Sciencedirect

Scheme 1 Reagents And Conditions A Novozym 435 40 8c Thf 24 H B Download Scientific Diagram

Preparation Of Optically Active 4 Substituted G Lactones By Lipase Catalyzed Optical Resolution In Heterocyclic Communications Volume 21 Issue 3 15
Www Tandfonline Com Doi Pdf 10 1080
Plos One Rutin Derivatives Obtained By Transesterification Reactions Catalyzed By Novozym 435 Antioxidant Properties And Absence Of Toxicity In Mammalian Cells



