Onset Temperature Dsc
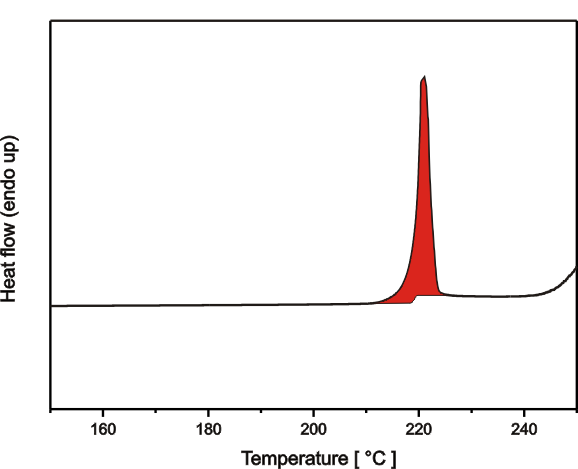
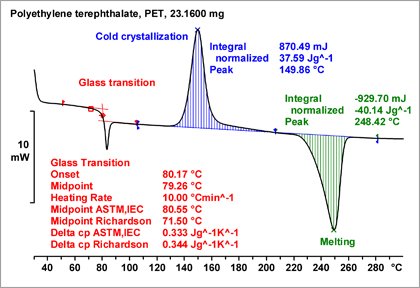
The DSC curve exhibits a small step at a midtemperature of about 74 °C, which is due to the glass transition, as well as a broad endothermic effect between about 160 and 280 °C, which is due to melting The melting temperature of a polymer is usually associated with the peak temperature, about 258 °C in this case.

Onset temperature dsc. 5 DSC Training Course 00 01 Heat Flow (W/g) 0 25 50 75 100 125 150 Exo Up Temperature (°C) Endothermic Heat Flow Heat flows into the sample as a result of either. Sample weight 28 mg;. The DSC onset temperature provides the temperature at which the reaction begins to progress The peak maximum represents the maximum rate of cure at the given heating rate condition The completion of the cure is reflected when the DSC response returns to linear behavior at the upper temperature end.
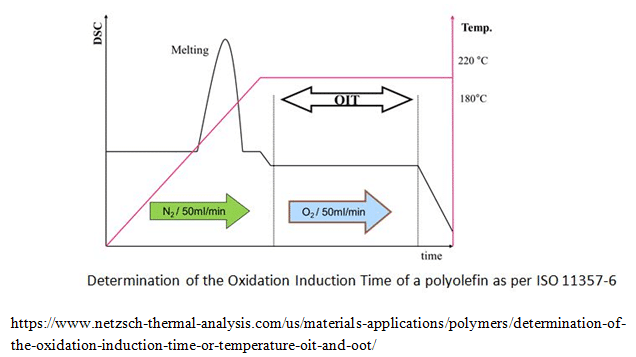
The DSC onset temperature has an expanded uncertainty (k= 2) of 0046 ºC Enthalpy of Fusion 2851 J·g–1± 019 J·g–1 The uncertainty was calculated as two times the square root of the sum of three components of variance Component one was the standard deviation of the mean from 14 measurements of the enthalpy of fusion of indium. The DSC onset temperature has an expanded uncertainty (k= 2) of 0046 ºC Enthalpy of Fusion 2851 J·g–1± 019 J·g–1 The uncertainty was calculated as two times the square root of the sum of three components of variance Component one was the standard deviation of the mean from 14 measurements of the enthalpy of fusion of indium. Useful variants of differential scanning calorimetry (DSC) include 1) heating with a temperature ramp in the presence of oxygen to determine the oxidation induction temperature (OITP), also known as the oxidation onset temperature (OOT), and 2) holding temperature constant in the presence of oxygen to determine the oxidation induction time (OIT).
Differential scanning calorimetry is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature Both the sample and reference are maintained at nearly the same temperature throughout the experiment Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time The reference sample should have. The thermal resistance between these two points is calculated as the onset slope of the heat flow versus temperature curve on the front side of the melting peak This is always a negative term Default is 000 mW/°C The onset slope constant is applicable to all Q Series DSC's, in the standard and modulated modes. When the temperature is stable at 560C a low exotherm can be observed, the DSC curve decreases slowly After the isotherm of 3 hours the same sample is cooled then heated a second time in the same conditions The difference between the two successive traces correspond to the sample transformation at 560C.
A dynamic DSC run can be carried out from room temperature to 500°C at a rate of 4 K/min in about 2 hours1 The instrument is popular for initial screening studies of exothermic reactions in thermal hazard evaluation laboratories1, 2. The DSC thermogram of the benzoxazine monomer exhibited an exothermic peak where onset and maximum temperatures were 162 and 215 °C, respectively This indicated that the curing of the benzoxazine monomer occurred in this temperature range. Isothermal temperature 5°C 2 Measurements DSC0 is used Measurement condition is 5mg of sample, isothermal temperature is 0°C, 5°C, 210°C, and 215°C In the Nitrogen and Oxygen atmosphere, the flow rate is 40mℓ/min Change from Nitrogen to Oxygen is automatically performed by the programming using gas controller unit 3 Results.
Why is the onset temperature on the DSC curve lower than the peak temperature?. DSC thermograms of the PVA electrospinning fibers are shown in Figure 3 The pure PVA fibers showed a relatively large and sharp endothermic curve with a peak at 196°C. For anodes, the decomposition and whole cell selfheating onset temperature is generally independent of SOC DSC exotherm onset temperatures of the anodes were generally within 10 °C of the onset of selfheating in whole cell ARC However, onset temperatures of the cathodes were typically observed above the ARC onset of whole cell runaway.
Measurement of the sample's transition temperature was made by heating the DSC cell from 50°C below the onset of the transition (to ensure that the material was in the desired phase prior to heating) to 30°C above the end of the transition Each crucible was subjected. Mean onset temperature °C, standard deviation 0024°C Note that these results reflect a general limitation of absolute temperature measurements with DSC instruments that are introduced by different heat transfer conditions in the samples. DSC–25) –167 – For shock sensitivity (SS) SS = log (Q DSC ) –072 x log(T DSC–25) –098 Q DSCis the energy of the exotherm in cal/g T DSCis the onset temperature of the exotherm in °C If EP or SS ≥0material is predicted to demonstrate the ability to propagate an explosion or be shock sensitive.
Users should not use the DSC to do this, as the furnace cannot be raised during a measurement or when the temperature exceeds 100 °C Reporting the temperature of the glass transition is, to some degree, based on preference No standard is currently established about which point along the curve is considered the official “temperature”. Figure 4 shows the crystallization peaks for DSC measurements at 10, 5, 2 and 1 K/min It can be seen that the onset (here “End”) as well as the peak temperature shift to higher temperatures as the cooling rate decreases Instead of an onset temperature of 1534°C at 10 K/min, the onset occurs already at 1616°C at 1 K/min. Onset point 1 925,2 °C Onset point 2 96 °C Enthalpy / J/g 16 (Endothermic effect) (340 1496) 9329°C 9781°C 12 Heat Flow (µV) Temperature (°C) Results A double peak of melting is monitored The onset peak of the first fraction is 925 C The top of the second fraction is 978 C The total heat of melting is 16 Jg1.
The melting temperature corresponds to the onset temperature of 1566 °C Four samples of different mass (0053 mg, 0846 mg, 3425 mg and 6301 mg) were measured at heating rates between 1 K/min and K/min. Heating rate 5 K/min. Enter the requested test parameters that will program the DSC to Equilibrate to a temperature below the onset of the literature melting temperature of the material Use the following equation to determine that temperature 5 min X Heating Rate (°C/min) = Temperature (°C).
Phenomena analysis for CHP derived from DSC and TAM From experimental results obtained from both DSC and TAM, two significant results are as follows Contaminants, such as H2SO4, NaOH, FeCl3, with CHP would increase degree of reaction hazards and decrease the exothermic onset temperature. Why is the onset temperature on the DSC curve lower than the peak temperature?. A dynamic DSC run can be carried out from room temperature to 500°C at a rate of 4 K/min in about 2 hours1 The instrument is popular for initial screening studies of exothermic reactions in thermal hazard evaluation laboratories1, 2.
The DSC is capable of cooling samples to 196°C through the use of liquid nitrogen Because of its low temperature (196°C), liquid nitrogen will cause tissue damage upon contact with your skin When you work with liquid nitrogen, use the following precautions • Liquid nitrogen boils rapidly when exposed to room temperature Only use liquid. In Figure 2a, the plots provide a leastsquares fit of the LTA onset measurements attained at the three heating rates to the DSC onset values attained at 10 °C/minute Every fit is good, with correlation coefficients larger than 099 The LTA measurements are likely to have positive offsets at all rates contrasted with the DSC onset measurement. 1) Hold for 10 min at 500°C 2) Cool from 500°C to 4500°C at 100°C/min 3) Hold for 10 min at 4500°C.
When you measure the melting point (Tm) in a DSC, you get not only the onset of melting, the Tm, but also the peak temperature, which corresponds to complete melting in organics and the energy that the melting transition needs in order to occur This is the enthalpy of the transitions, and it is associated with the crystallinity of materials. Differential scanning calorimetry (DSC) monitors heat effects associated with phase transitions and chemical reactions as a function of temperature In a DSC the difference in heat flow to the sample and a reference at the same temperature, is recorded as a function of temperature The sample is sealed in an aluminum pan. The thermal resistance between these two points is calculated as the onset slope of the heat flow versus temperature curve on the front side of the melting peak This is always a negative term Default is 000 mW/°C The onset slope constant is applicable to all Q Series DSC's, in the standard and modulated modes.
Standard reference was found to present a temperature of onset of melting point of °C Regarding the samples of active agents provided by the different laboratories, a variation ranging from to °C was measured In terms of DH m, the samples presented an average value of 3112 kJ mol1 Uniterms Zidovudine/purity determination. The Onset temperature is calculated by adjusting the lines to find the tangent point where the slope changes The calculated temperature will be noted as the Wax Appearance Temperature Results Once the DSC has completed the test, a graph will be produced It will require the operator to take a more in depth. 12 This test method also determines the extrapolated onset temperature and peak heat flow temperature for the exothermic reaction 13 This test method may be performed on solids, liquids or slurries 14 The applicable temperature range of this test method is 25 to 600°C 15 The values stated in SI units are to be regarded as standard.
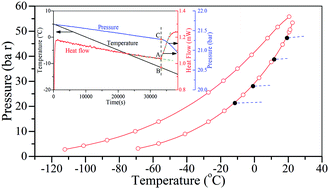
Which to conduct DSC measurements With the highpressure DSC 4 HP Phoenix®, thermal effects in a sample can be analyzed in the pressure range from vacuum to 15 MPa (150 bar) and at temperatures from 150°C to 600°C, depending on the type of gas DSC 4 HP Phoenix® DSC 3500 Sirius DSC 214 Polyma. Polyethylene Oxidation Onset Temperature °C °C10 0 10 Heat Flow (mW) 50 100 150 0 250 300 Temperature (°C) Oxidation Onset Temperature (OOT). The DSC is capable of cooling samples to 196°C through the use of liquid nitrogen Because of its low temperature (196°C), liquid nitrogen will cause tissue damage upon contact with your skin When you work with liquid nitrogen, use the following precautions • Liquid nitrogen boils rapidly when exposed to room temperature Only use liquid.
For example, with pure substances, the onset temperature is the melting temperature Evaluation and Interpretation of Peak Temperatures of DSC Curves Part 1 Basic Principles However, if peaks are broad, the onset temperature cannot be precisely determined and it loses its physical meaning. The onset temperature is the beginning of the deviation from the baseline measured by some quantitative measure as described in 243 The peak signal is the maximum deviation of the signal (Δyvalue) from the baseline and the peak temperature is the temperature (xvalue) corre sponding to the peak signal. We have access to a higher temperature DSC/DTA instrument capable of a maximum temperature of 1500°C, though it has a lower sensitivity at temperatures below 725°C compared to our primary DSC* Pans of Al, Cu, Au, Pt, alumina, and graphite are available and need to be chosen to avoid reactions with samples and with regard to the temperature range of the measurement.
The Oxidation Onset Temperature (OOT), obtained by intersecting the extrapolation of the DSC signal relative to the exothermic oxidation event by the DSC signal baseline, decreased as the volume concentrations of biodiesel increased. The dynamic method involves heating of the sample under oxidizing conditions at a defined constant heating rate until the onset of the reaction The corresponding OxidativeInduction Temperature, also known as Oxidation Onset Temperature (OOT), is equivalent to the extrapolated onset temperature of the exothermal DSC effect taking place. 1) Hold for 10 min at 500°C 2) Cool from 500°C to 4500°C at 100°C/min 3) Hold for 10 min at 4500°C.
When the liquids are mixed at °C, the 5minute type starts curing after 5minutes and the 30minute type starts curing after 30 minutes For the measurements, a DSC2 high sensitivity differential scanning calorimeter was connected to a SSC50H Disk station The sample weight was 10mg and an Al open pan was used. The magnetic change as a secondorder transition appears in the reversing part (black dashed curve), whereas the structural change becomes evident in the nonreversing part (red dashed curve), with an extrapolated onset temperature of 756°C Figure 5 TMDSC measurement on steel (heating rate 5 K/min, period 60 s, amplitude 05 K) blue total heat flow, red nonreversing curve, black reversing curve. Company from their historical data bank It shows that in many cases, the “onset temperature” detected by DSC can be as much as 50 oC higher than that reported from adiabatic instruments In fact in a substantial number of instances the difference between to two methods is as much as 100 oC (There are several cases where the DSC determined “onset.
We have access to a higher temperature DSC/DTA instrument capable of a maximum temperature of 1500°C, though it has a lower sensitivity at temperatures below 725°C compared to our primary DSC* Pans of Al, Cu, Au, Pt, alumina, and graphite are available and need to be chosen to avoid reactions with samples and with regard to the temperature range of the measurement. The NCA anode decomposition evident by TRXRD is consistent with the NCA anode DSC onset temperature for SEI decomposition at 100% SOC (~100 °C, Fig 3C) However, the NCA cells exhibited an unusually high ARC T onset of 133 °C One possible explanation is that SEI decomposition occurs shortly before the onset of anode decomposition, and it is the anode decomposition which provides more heat for the onset of the whole cell selfheating. DSC onset temperature provides the temperature at which the reaction begins to progress The peak maximum represents the maximum rate of cure at the given heating rate condition The completion of the cure is reflected when the DSC response returns to linear behavior at the upper temperature end If the area under the exothermic peak is integrated, this.
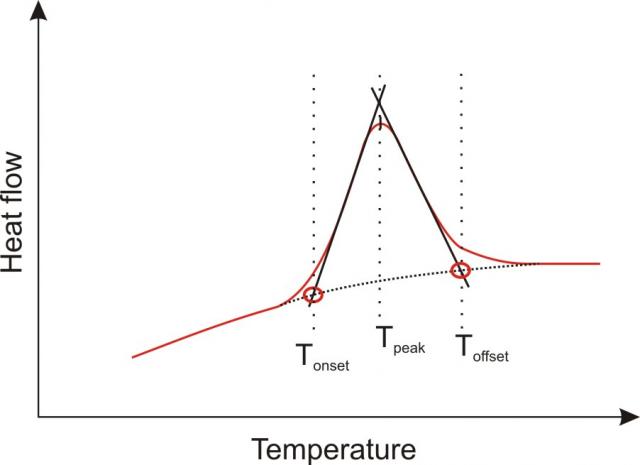
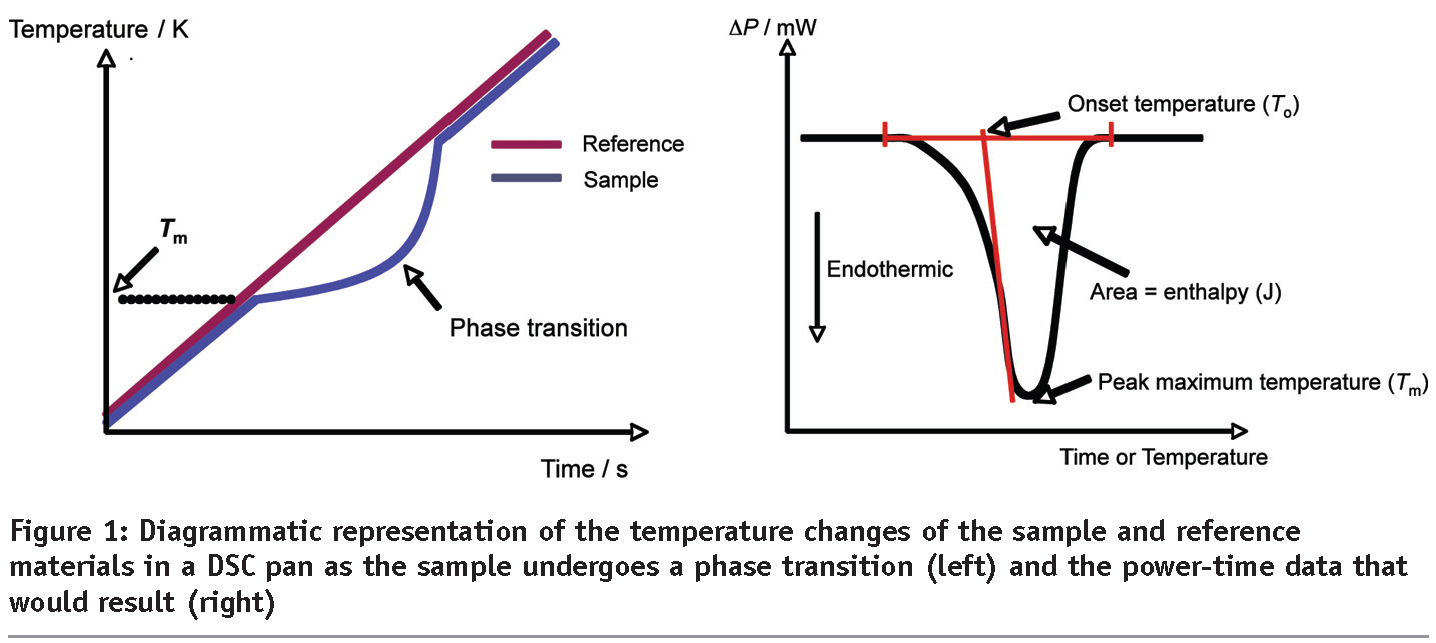
DSC thermogram with definitions of onset temperature (Tonset), peak temperature (Tpeak) and offset temperature (Toffset) Any peak in the thermogram is characterised by a peak temperature, the onset and the offset temperature The peak temperature is obviously the temperature at the maximum/minimum of the thermal event. This exothermal effect can be described by different characteristic temperatures The Onset temperature is the extrapolated beginning temperature of the reaction;. The Onset is the intersection between the baseline and the tangent at the point of the highest slope The baseline of the curves you will get in origin probably correct The point of the maximal slope is the maximum of the 1st derivate and the slope is the value at this maximum So you have to calculate the tangent at this point and its.
E0908(14)e1 Standard Test Methods for Oxidation Onset Temperature of Hydrocarbons by Differential Scanning Calorimetry oxidation onset temperature~ oxidative stability~ pressure differential scanning calorimetry~ hydrocarbons~ Products and Services / Standards & Publications / Standards Products. Fig 2 Temperature scan, converted to partial molar heat capacity, and fi tted baseline for 1 mg mL1 hen egg white lysozyme in 02 M glycine buffer, pH 27 Data obtained at a scan rate of 1 o C/min using a CSC 6100 NDSC II H C dT T T ∆ =∫ p 2 1 S C T dT T T ( p /) 2 1 ∆ =∫ 0 10 30 40 50 60 70 80 90 30 40 50 60 70 80 90. The Onset temperature is calculated by adjusting the lines to find the tangent point where the slope changes The calculated temperature will be noted as the Wax Appearance Temperature Results Once the DSC has completed the test, a graph will be produced It will require the operator to take a more in depth.
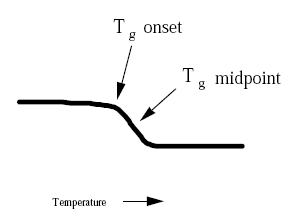
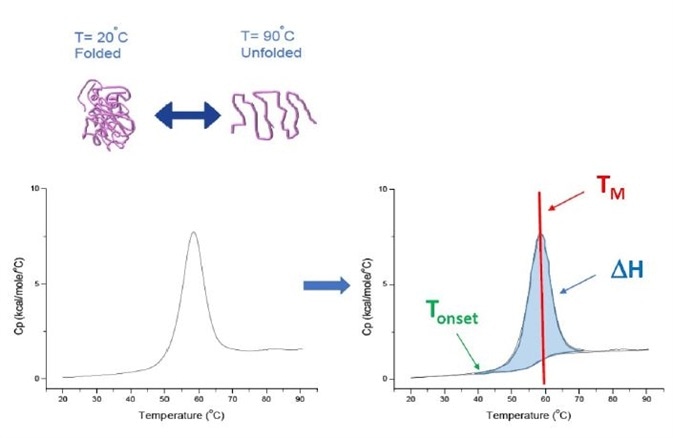
The End temperature is the extrapolated end temperature of the reaction Figure 1 DSC 214 Polyma measurement;. A true transition temperature to Tg In fact, there are actually 5 accepted ways in which the Tg of a material can be assigned by DSC, as is represented in the following figure Tb represents the very start of the detection of the change in heat flow or heat capacity at Tg, T1 is the onset temperature, Tg is the midpoint temperature and the most. The HOBO MX22 waterproof temperature/light level logger leverages the power of Bluetooth Low Energy (BLE) to deliver accurate temperature and lightlevel measurements straight to your mobile device with Onset's free HOBOconnect app Designed for durability, this compact waterproof logger is ideal for measuring temperature in streams, lakes, oceans, and soil environments.
In addition, the standard reference was found to present a temperature of onset of melting point of °C Regarding the samples of active agents provided by the different laboratories, a variation ranging from to °C was measured In terms of DH. Onset temperature provided the liquidus at equilibrium Onset temperatures determined by the tangent method and at heating rates of 051–101C/min were used in the second case The use of the onset temperature was justified by the fact that pure (stoichiometric) referencegrade compounds are used Nevertheless,. The dynamic method involves heating of the sample under oxidizing conditions at a defined constant heating rate until the onset of the reaction The corresponding OxidativeInduction Temperature, also known as Oxidation Onset Temperature (OOT), is equivalent to the extrapolated onset temperature of the exothermal DSC effect taking place The isothermal technique involves heating of the sample under a protective gas, followed by maintaining the sample at constant temperature under protective.
Onset Temperature The extrapolated onsettemperature (according to DIN EN ISO ) is the designed intersection point of the extrapolated baseline and the inflectional tangent at the beginning of the melting or crystallization peak The baseline and the inflectional tangent are determined from the temperaturedependent heat flow signal. Differential scanning calorimetry (DSC) monitors heat effects associated with phase transitions and chemical reactions as a function of temperature In a DSC the difference in heat flow to the sample and a reference at the same temperature, is recorded as a function of temperature The sample is sealed in an aluminum pan. Onset Temperature Defined to be the temperature at which the heat that is released by a reaction can no longer be completely removed from the reaction vessel, and consequently, results in a detectable temperature increase The onset temperature depends on detection sensitivity, reaction kinetics, on vessel size and on cooling, flow and agitation characteristics.

Oxidative Stability Of Fats And Oils Measured By Differential Scanning Calorimetry For Food And Industrial Applications Intechopen

Find Onset And Peak Temperatures Mathematica Stack Exchange
Www Osti Gov Servlets Purl
Onset Temperature Dsc のギャラリー
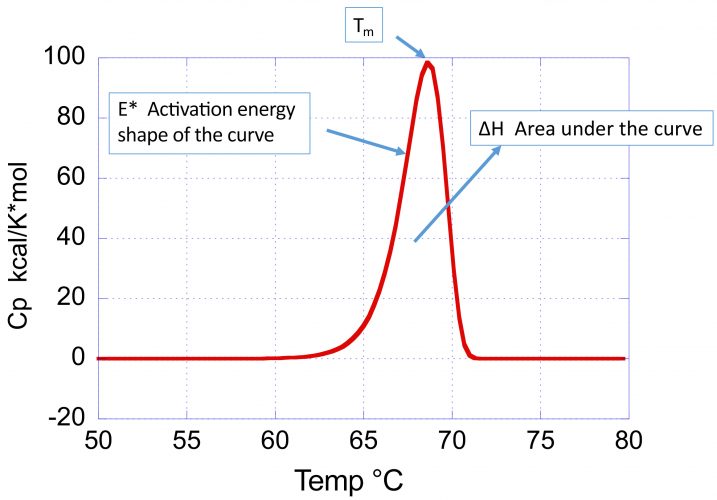
Can Dsc Calorimetry Gauge Long Term Stability For Monoclonal Antibodies

Moving Dsc Downstream Exploiting Differential Scanning Calorimetry As A Process Development Tool Bioprocess Internationalbioprocess International

Raw Material Identification With Dsc
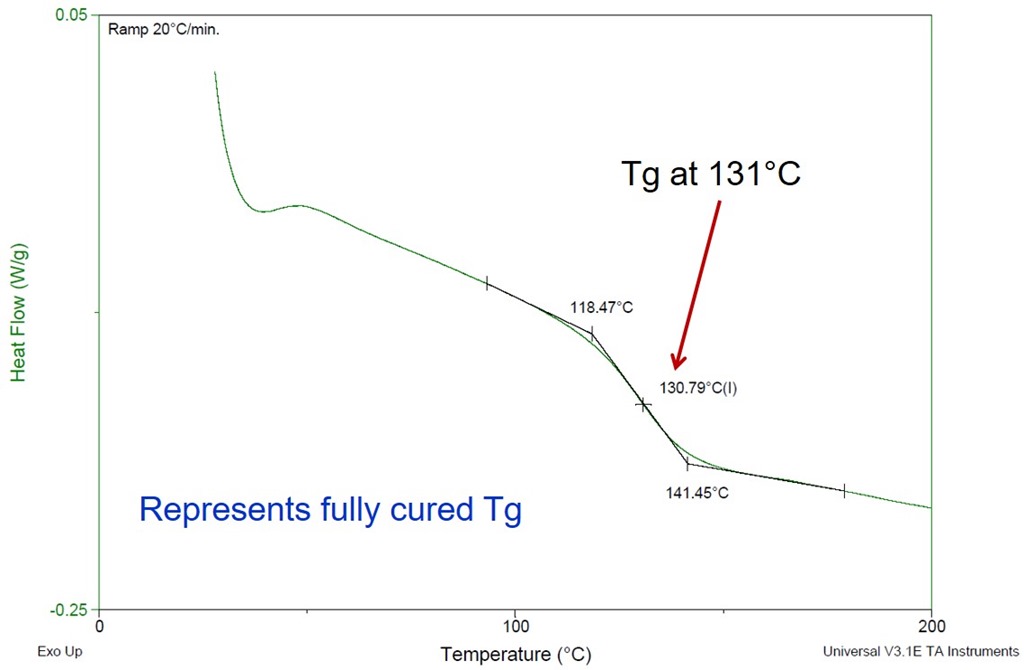
Practical Tips For Curing Thermosets Part Two Impact Of Cure Temperature On The Glass Transition Temperature Polymer Innovation Blog
Http Www Lamav Ufscar Br Artpdf Jacs93b Pdf
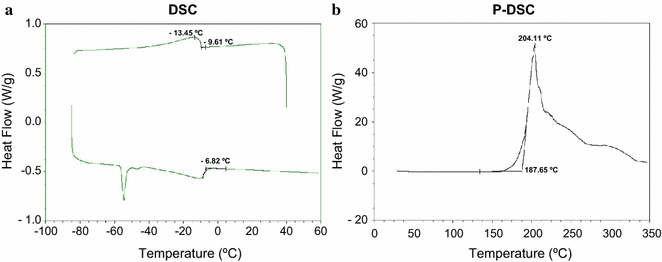
High Cell Density Production Of Multimethyl Branched Long Chain Esters In Escherichia Coli And Determination Of Their Physicochemical Properties Biotechnology For Biofuels Full Text

Reaction Temperature And Reaction Enthalpy Netzsch Analyzing Testing
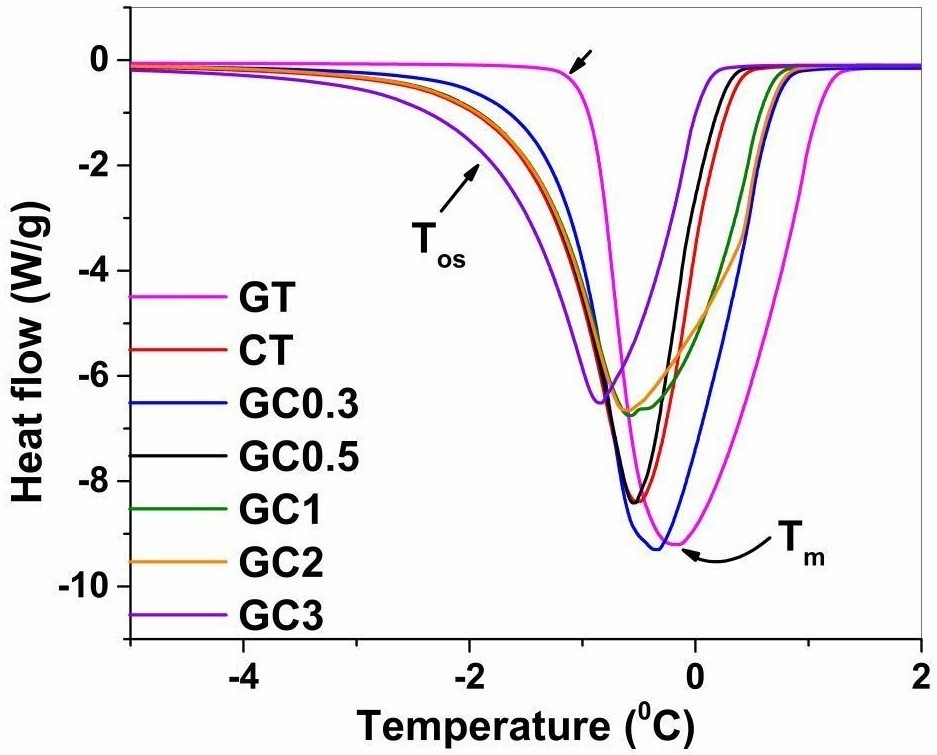
Porosity And Dielectric Properties As Tools To Predict Drug Release Trends From Hydrogels Springerplus Full Text
Dsc Hydrates

Differential Scanning Calorimetry Netzsch Thermal Academy

Monitoring The Polymorphism Of Paracetamol By Means Of Dsc

Determining T Onset T P T Endset Of Heat Flow Versus Temperature In Download Scientific Diagram

Differential Scanning Calorimetry Wikipedia
Q Tbn And9gcrcldg N Qm Nvicnfpbnzk6cxpjjmyr58 Fpzchaq5xqhutiew Usqp Cau
Http Lamav Weebly Com Uploads 5 9 0 2 Understanding Glass Through Differential Scanning Calorimetry Compactado Pdf

Multi Scale Thermal Stability Study Of Commercial Lithium Ion Batteries As A Function Of Cathode Chemistry And State Of Charge Sciencedirect

Melting Temperatures And Enthalpies Netzsch Analyzing Testing

Determination Of The Melting Temperature Heat Of Fusion And Purity Analysis Of Different Samples Of Zidovudine Azt Using Dsc
Ejpau 05 Glass Transition In Food Industry Characteristic Properties Of Glass Transition And Determination Techniques

Iso 1 16 En Plastics Differential Scanning Calorimetry Dsc Part 1 General Principles
Www Perkinelmer Com Cmsresources Images 44 gde Dscbeginnersguide Pdf
Www Perkinelmer Com Cmsresources Images 44 gde Dscbeginnersguide Pdf
Novel Isochoric Measurement Of The Onset Of Vapor Liquid Phase Transition Using Differential Scanning Calorimetry Physical Chemistry Chemical Physics Rsc Publishing
Http Docs Tainstruments Com Training 1904 Dsc Pdf
Dsc Hydrates

Schematic Illustration Of Transition Temperatures T O Onset Download Scientific Diagram

Handbook Of Food Engineering
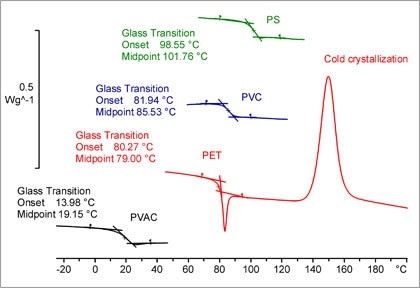
Determination Of Glass Transition Temperature
Polymerscience Physik Hu Berlin De Docs Manuals Dsc Pdf

Determination Of The Melting Temperature Heat Of Fusion And Purity Analysis Of Different Samples Of Zidovudine Azt Using Dsc
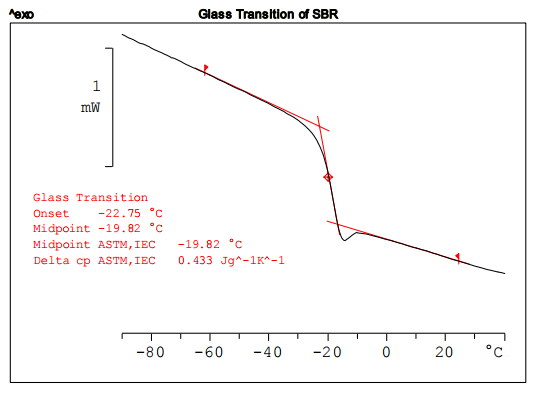
Measurement Of The Glass Transition Temperature With Dsc Mettler Toledo

Onset Temperature An Overview Sciencedirect Topics
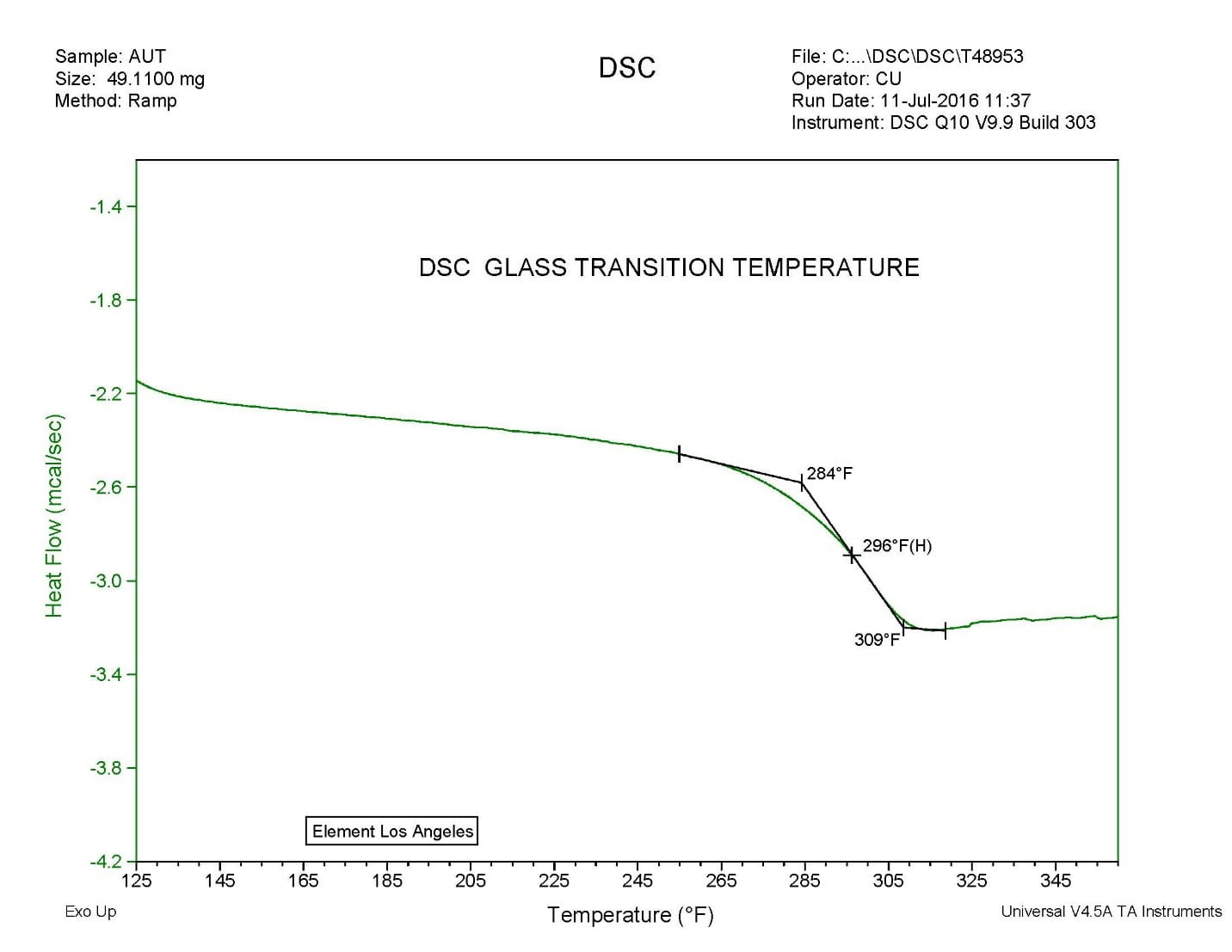
Considerations For Measuring Glass Transition Temperature Element

Differential Scanning Calorimetry Testing Dsc Analysis Microtrace

In Dsc Graph Of Lipids Which Point Describes Melting Point More Appropriately
Fast Scan Differential Scanning Calorimetry European Pharmaceutical Review
Dsc Analysis Fundamentals And Applications

Dsc Astm E1269
1 Discuss The Differences Between The Melting Pro Chegg Com
Using Differential Scanning Calorimetry For Biosimilarity And Batch To Batch Comparability
Differential Scanning Calorimetry Oxidative Induction Time Dsc Oit Analytical Techniques Jordi Labs Laboratory Testing

Glass Transition Temperature Tg Of Plastics Definition Values
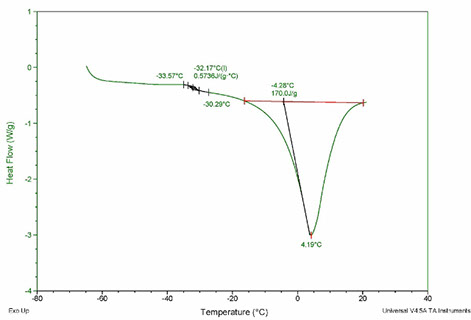
Low Temperature Thermal Analysis Lt Ta Lyophilization Services

Within Established Test Methods Such As Dsc Which Number Should Be Download Scientific Diagram

Dsc Interpretaion And Application
3
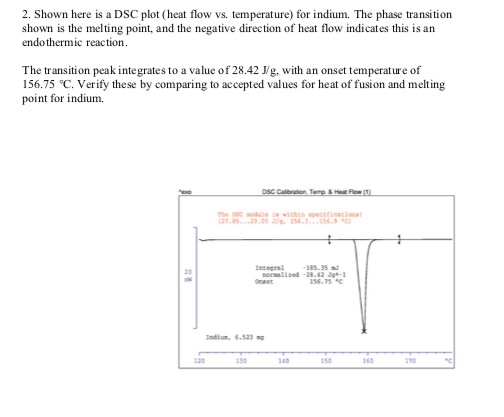
Solved 2 Shown Here Is A Dsc Plot Heat Flow Vs Tempera Chegg Com
Www Osti Gov Servlets Purl
Www Iitk Ac In Che Pdf Resources Tga Dsc Reading Material Pdf

Differential Scanning Calorimetry Netzsch Thermal Academy
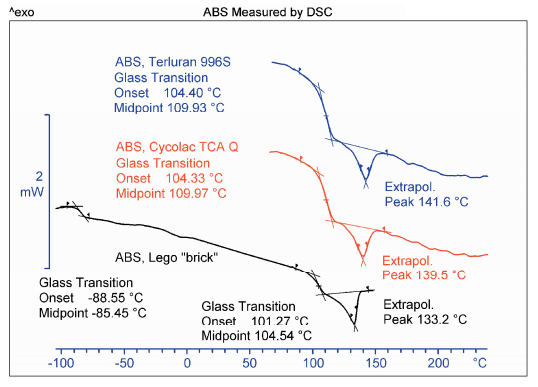
Abs Glass Transition By Dsc Mettler Toledo

From Each Dsc Derived Endotherm Endotherm Onset Temperature Tonset Download Scientific Diagram

Application Of Differential Scanning Calorimetry To The Characterization Of Biopolymers Intechopen
Depot Ceon Pl Bitstream Handle 6 Chrostek Pdf Sequence 1 Isallowed Y
Q Tbn And9gcr4mcxoo1go7m2mva8ehafazx9c8qzyt2heekxifr3tr9ux3cyz Usqp Cau

Differential Scanning Calorimetry

Verification Of Dsc Termperature Enthalpy Youtube
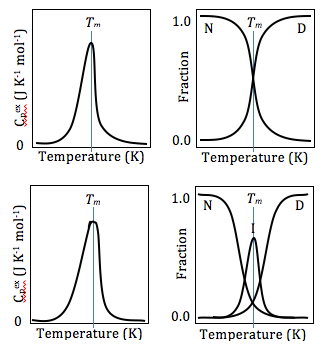
Differential Scanning Calorimetry Wikipedia

Iso 1 09 En Plastics Differential Scanning Calorimetry Dsc Part 1 General Principles
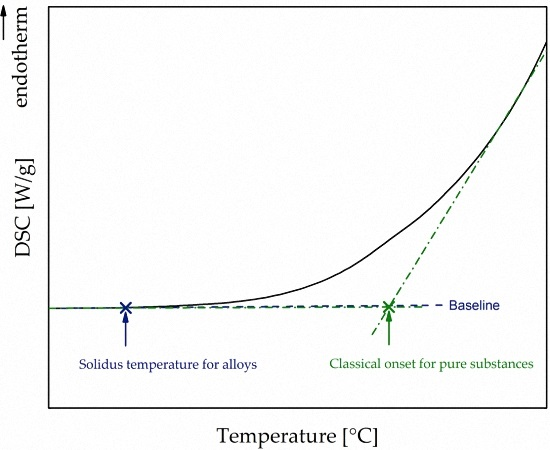
Metals Free Full Text Differential Scanning Calorimetry And Thermodynamic Predictions A Comparative Study Of Al Zn Mg Cu Alloys Html
Http Www Tainstruments Com Pdf Literature Ta039 Pdf
Http Lamav Weebly Com Uploads 5 9 0 2 Understanding Glass Through Differential Scanning Calorimetry Compactado Pdf
.jpg)
Using Dsc For The Identification Of Polymorphism
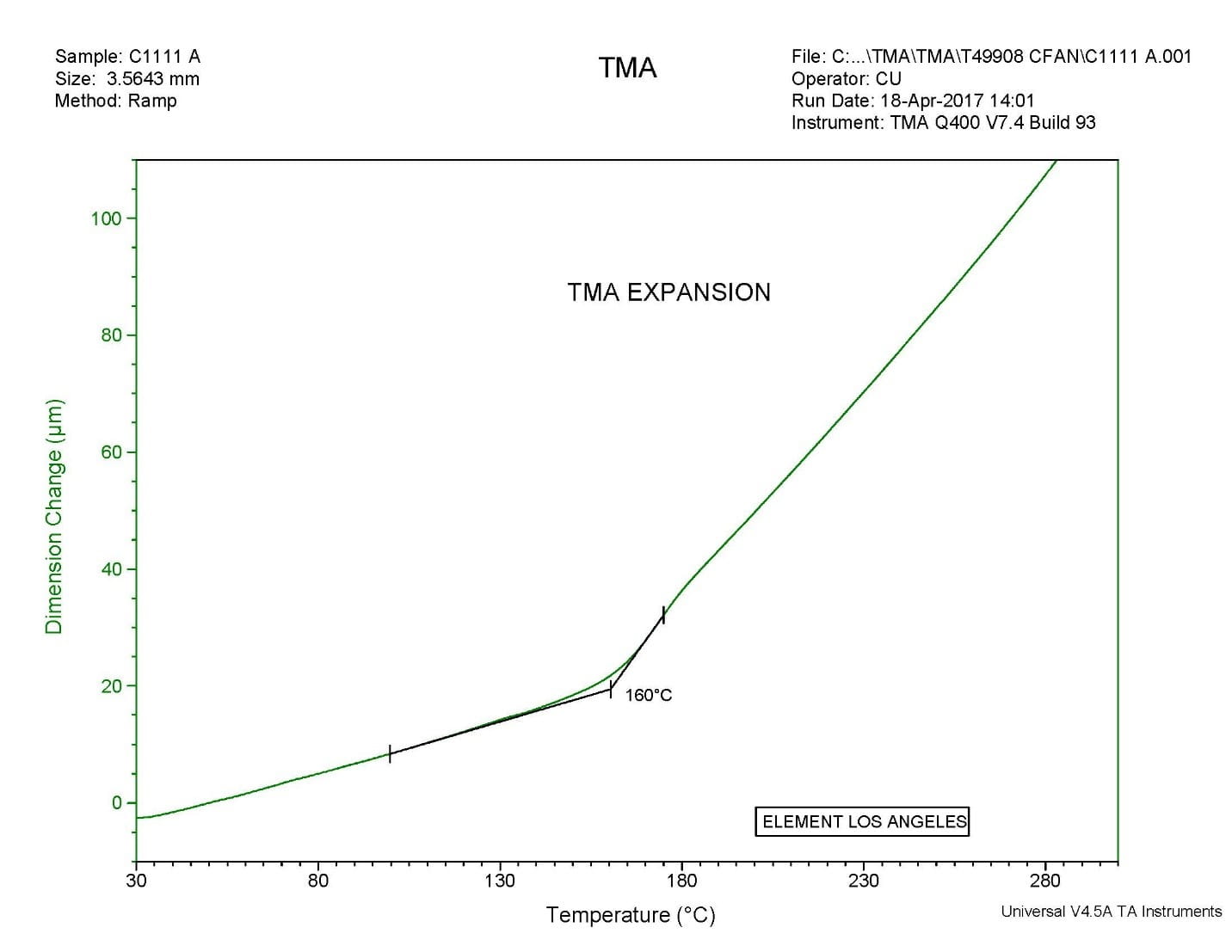
Considerations For Measuring Glass Transition Temperature Element
Http Www Sapub Org Global Showpaperpdf Aspx Doi 10 5923 J Ajps 01
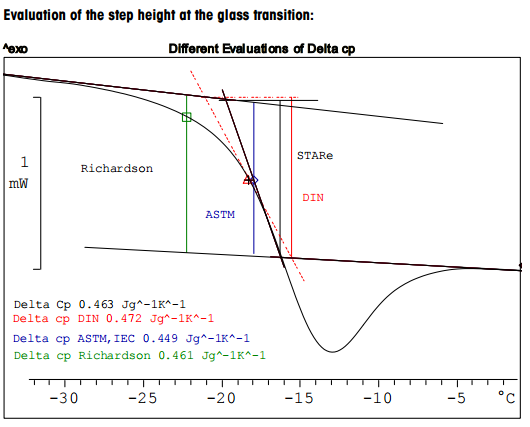
Evaluation Possibilities For The Glass Transition Mettler Toledo
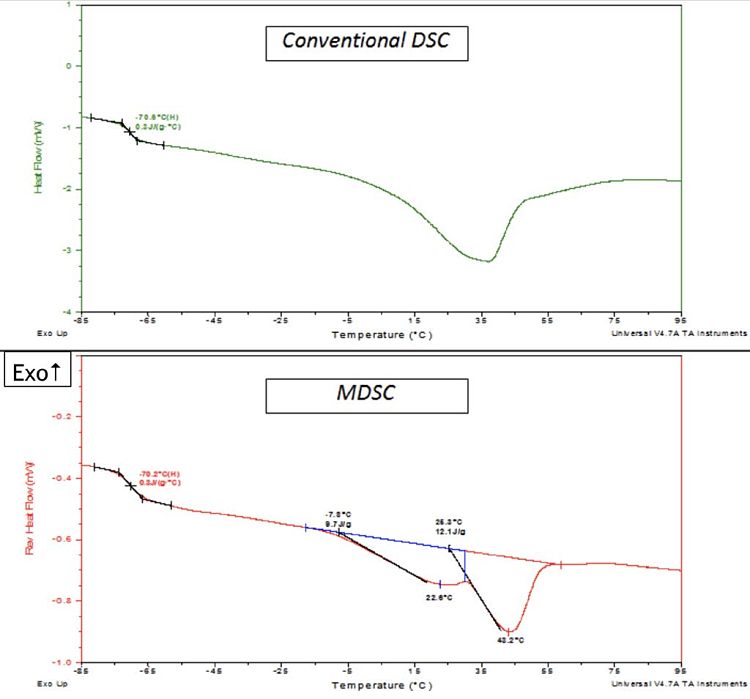
Dsc Analysis Of Polymers Thermal Eag Laboratories
Http Dx Doi Org 10 Aphyspola 131 1315

Glass Transition Temperature Netzsch Analyzing Testing
Evaluation And Interpretation Of Peak Temperatures Of Dsc Curves Part 1 Basic Principles Mettler Toledo

Oxidative Induction Time Oit And Oxidative Onset Temperature Oot Netzsch Analyzing Testing
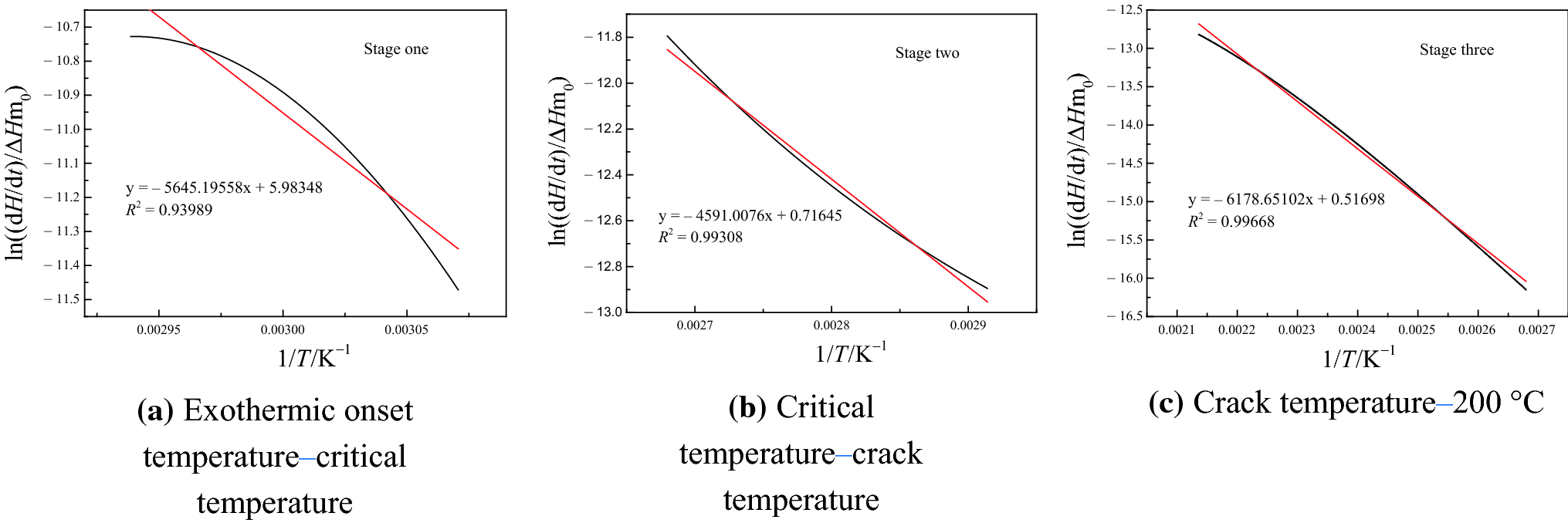
Figure 12 Macrocharacteristics Of Gaseous Indicator Products And Exothermicity During Low Temperature Oxidation Of Samples From Different Regions Of The Same Coal Seam From Huainan Anhui China Springerlink

Schematic Diagram Of A Dsc Curve To Show The Onset Temperature To Download Scientific Diagram
Themedicinemaker Com Fileadmin White Papers 005 0017 Malvern White Paper Pdf
Www Mdpi Com 1996 1944 13 4486 Pdf
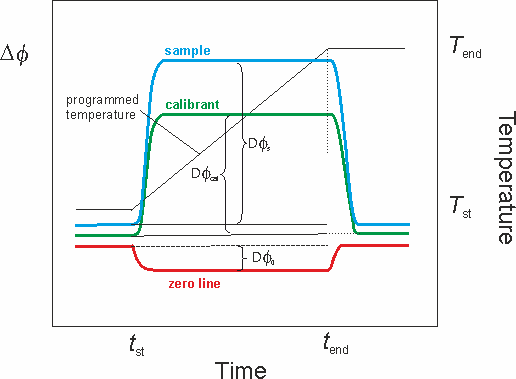
Molecular Energetics Group
Q Tbn And9gcrbmakca2remtqoezbxzvw K4xifsrv8wmq6lfrrdi Usqp Cau

Oxidative Induction Time Oit And Oxidative Onset Temperature Oot Netzsch Analyzing Testing

Differential Scanning Calorimetry Dsc
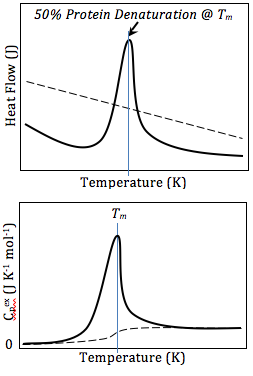
Differential Scanning Calorimetry Wikipedia
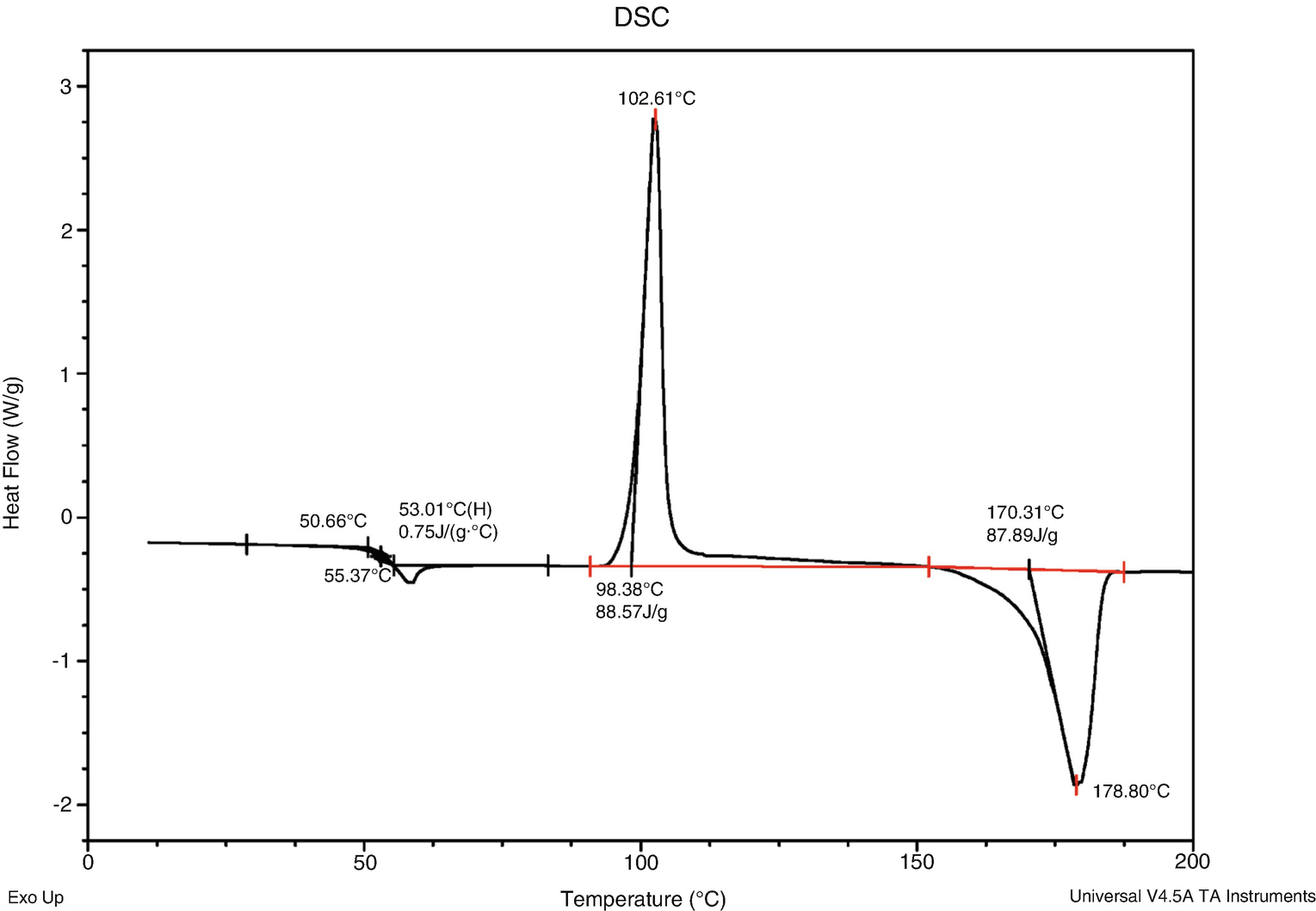
Thermal Analysis Springerlink
Http Iopscience Iop Org Article 10 10 1757 9x 226 1 Pdf
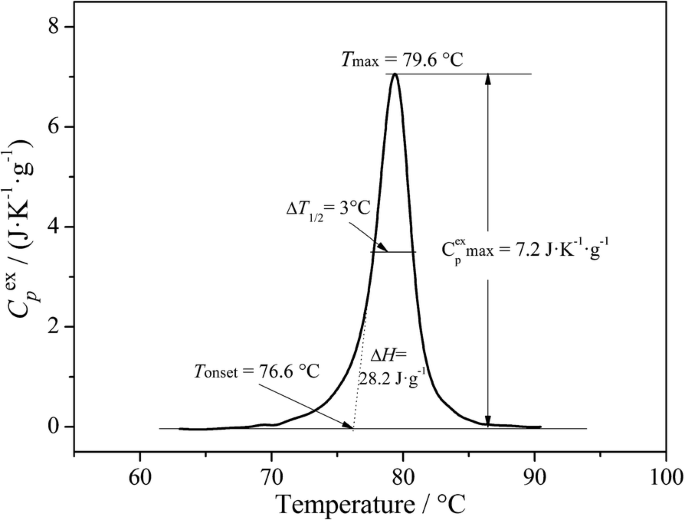
Micro Differential Scanning Calorimetry And Micro Hot Table Method For Quantifying Deterioration Of Historical Leather Heritage Science Full Text

Melting Peak An Overview Sciencedirect Topics

Figure 2 From Effect Of Milling On Dsc Thermogram Of Excipient Adipic Acid Semantic Scholar

Methods Of Thermal Analysis As A Tool To Develop Cryopreservation Protocols Of Vegetatively Propagated Crops Intechopen

Glass Transition Temperature Netzsch Analyzing Testing

Dsc Curves Of Water At Different Pressures The Onset Temperature Of Download Scientific Diagram
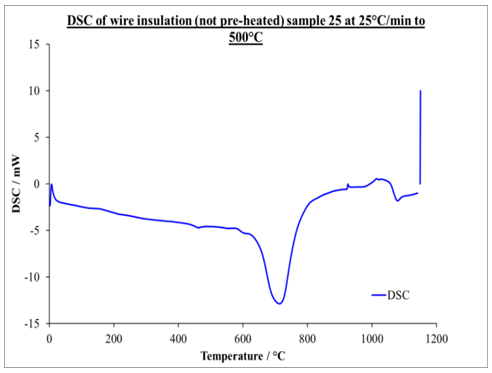
Application Of Dsc In The Study Of Thermal History Of Cable Insulation Wire

Norbiolab No Uncategorized

A Typical Dsc Thermogram Showing Freezing Onset Temperatures T Het And Download Scientific Diagram
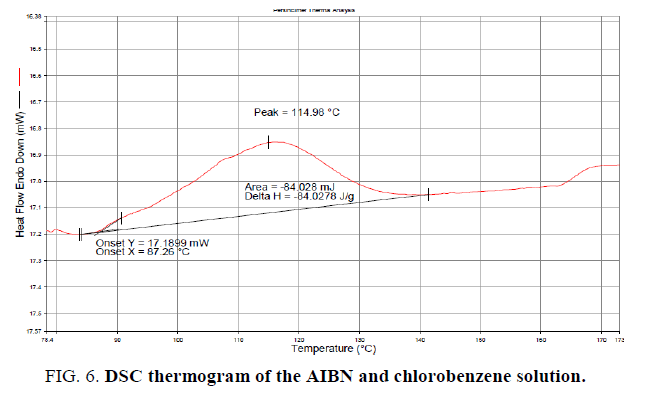
Reaction Calorimetry As A Tool For Thermal Risk Assessment And Improvement Of Safe Scalable Chemical Processes
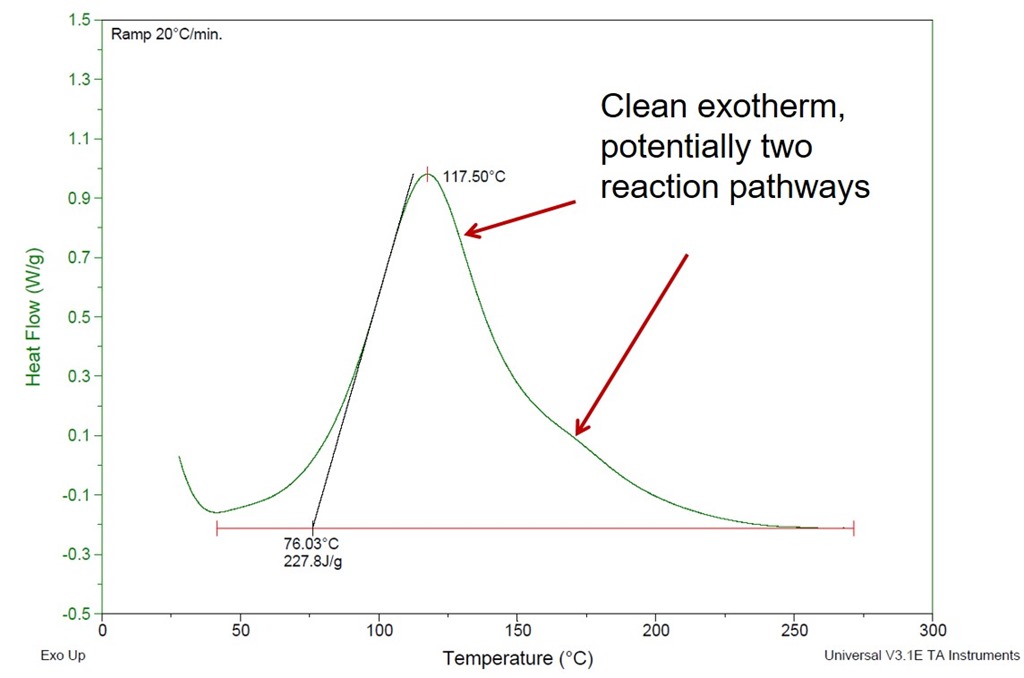
Practical Tips For Curing Thermosets Part Two Impact Of Cure Temperature On The Glass Transition Temperature Polymer Innovation Blog

Onset Temperature An Overview Sciencedirect Topics
Monitoring Of Pentoxifylline Thermal Behavior By Novel Simultaneous Laboratory Small And Wide X Ray Scattering Swaxs And Differential Scanning Calorimetry Dsc
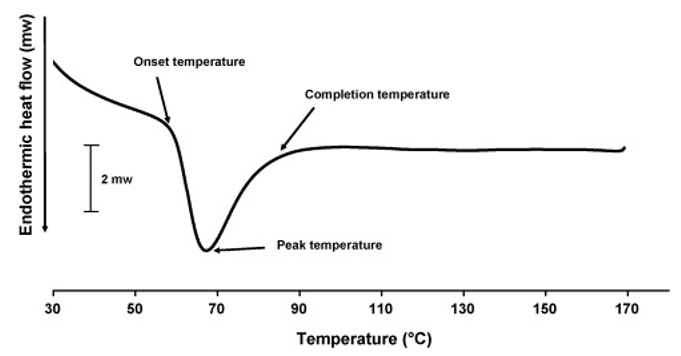
Gelatinization Temperature Determination Creative Proteomics

A Review On Differential Scanning Calorimetry Technique And Its Importance In The Field Of Energetic Materials In Physical Sciences Reviews Volume 3 Issue 4 18
Differential Scanning Calorimetry Dsc Thermogram Of Compound 4 With Download Scientific Diagram
2
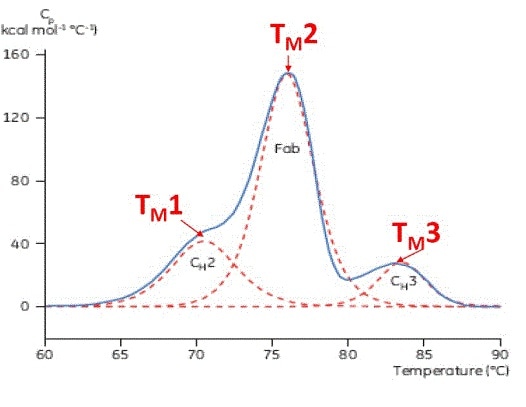
Using Differential Scanning Calorimetry For Biosimilarity And Batch To Batch Comparability
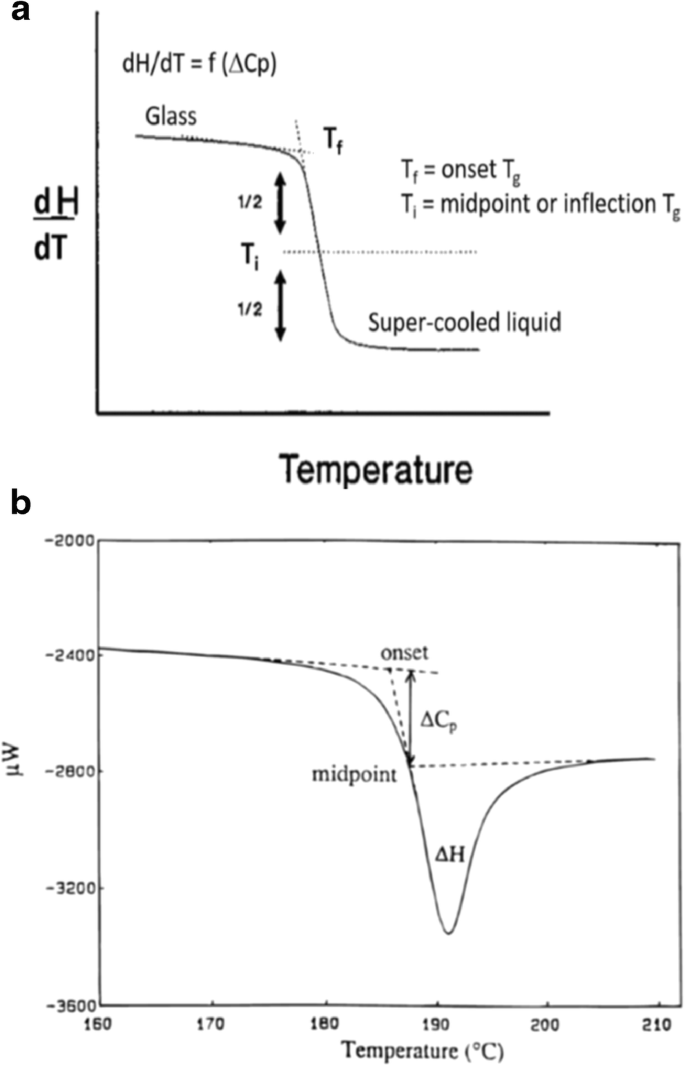
Commentary Considerations In The Measurement Of Glass Transition Temperatures Of Pharmaceutical Amorphous Solids Springerlink



