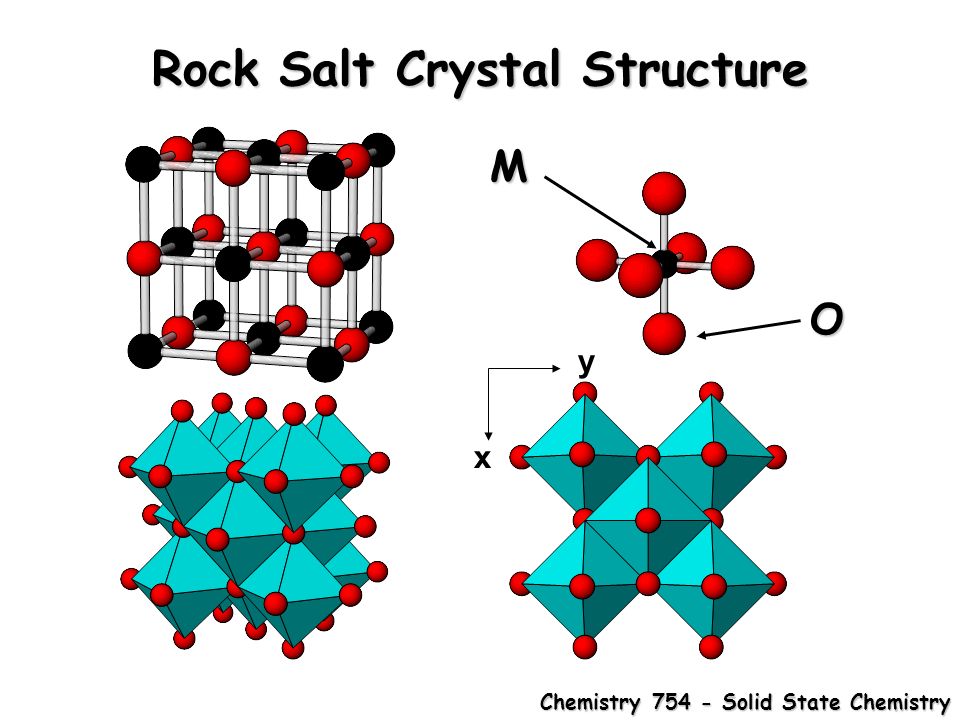
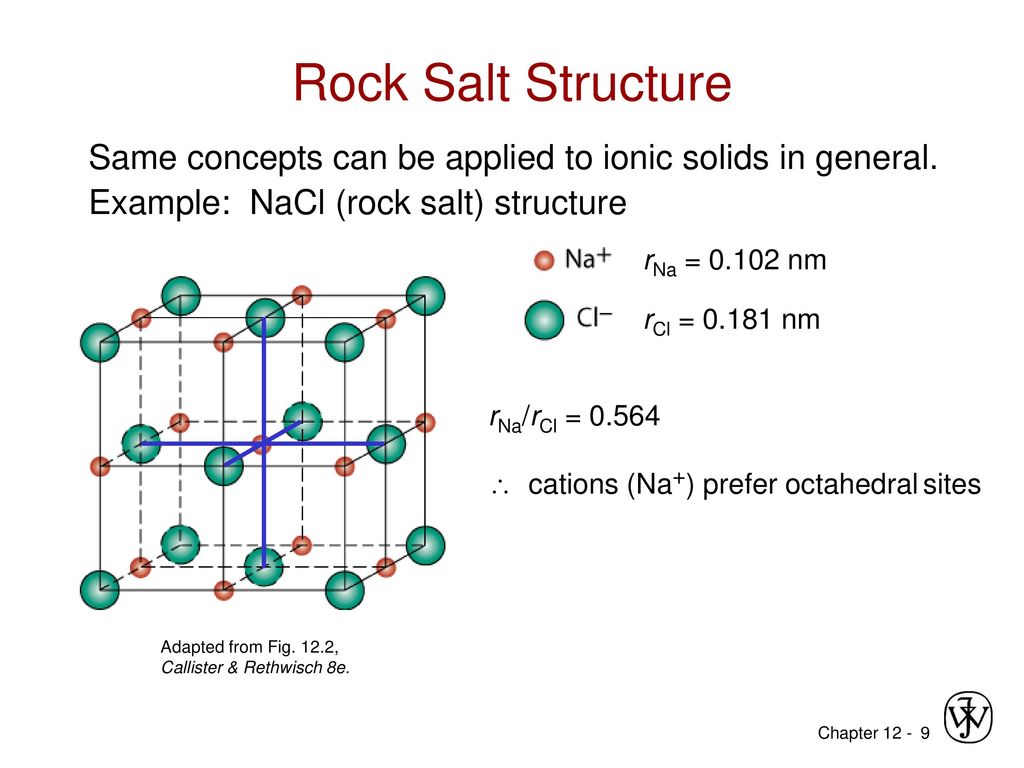
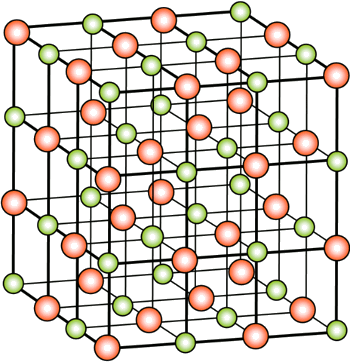
Rock Salt Structure
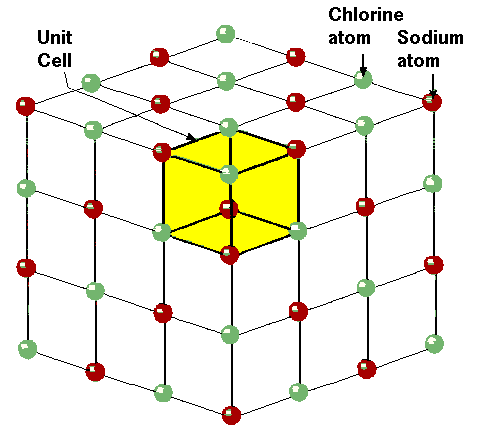
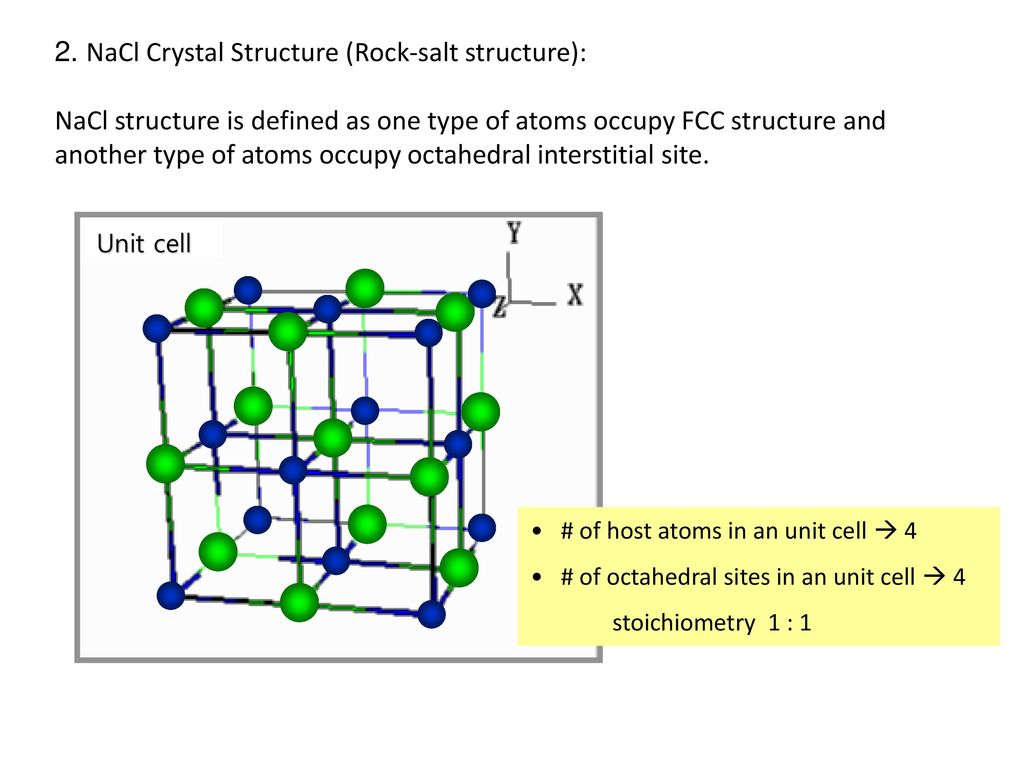
The Sodium Chloride Structure General Notes Halite, rock salt, sea salt, table salt, salt Isostructural Compounds MgO, TiO, TiC, LaN, NaI KCl, RbF, AgCl, SrS Structure Fig 1 A single unit cell of NaCl Fig 2 A 3x3x3 lattice of NaCl Shown below are two crystallographic planes in NaCl Notice that the (111) plane is hexagonally.

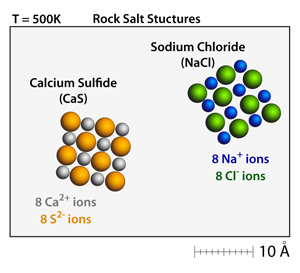
Rock salt structure. This is the same material that makes up the tiny crystals of table salt The cubic arrangement of atoms, and the cubes that are visible in these large Halite crystals and the small grains of table salt, is called the rock salt structure. "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement The symmetry is the same as that of methane The sulfur atom is in the 6 oxidation state while the four oxygen atoms are each in the −2 state. Rock salt also known as NaCl is an ionic compound It occurs naturally as white cubic crystals The structure of NaCl is formed by repeating the unit cell It has an organized structure and has a 11 ratio of NaCl.
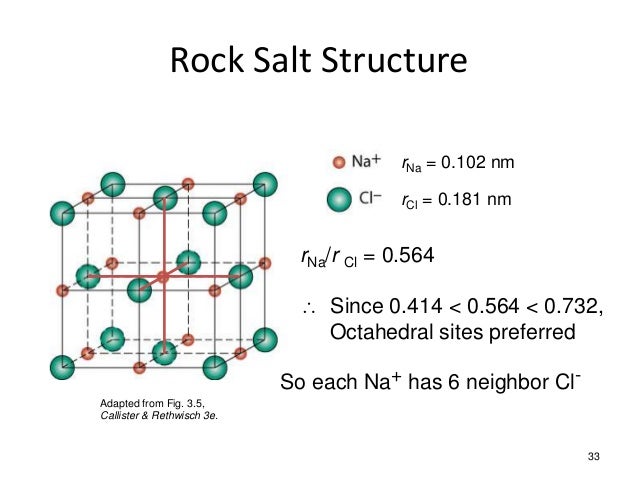
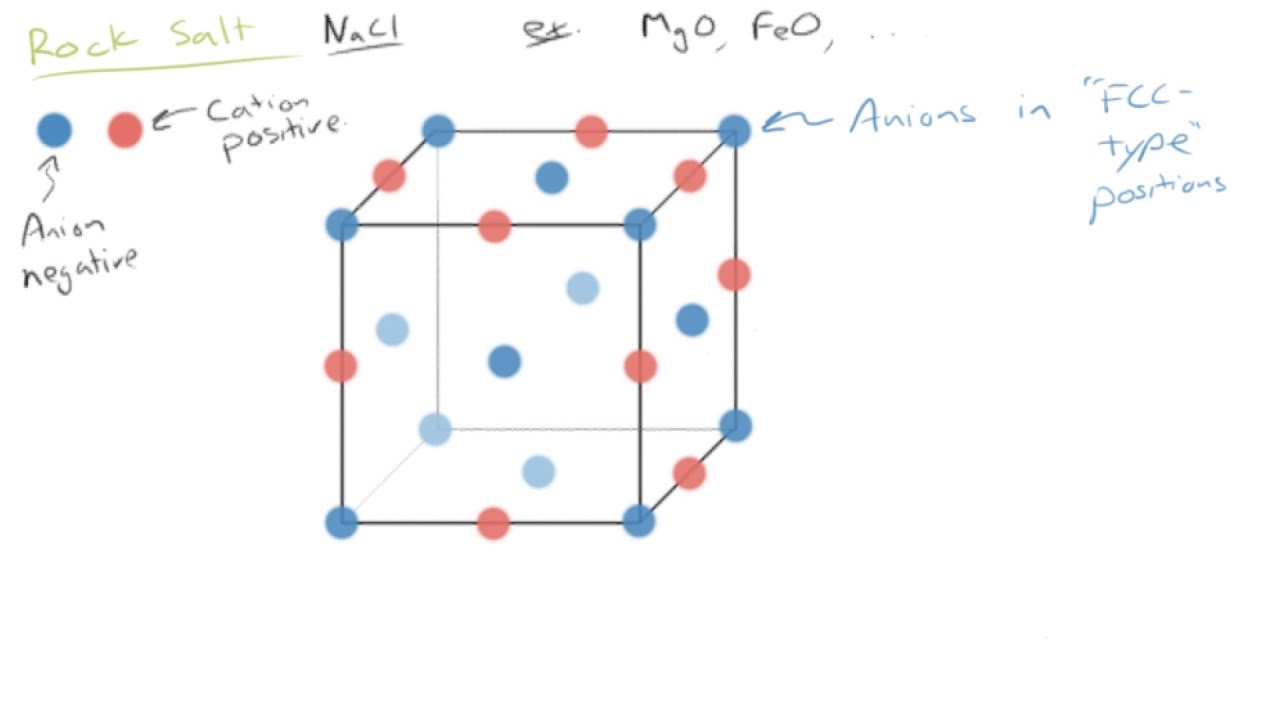
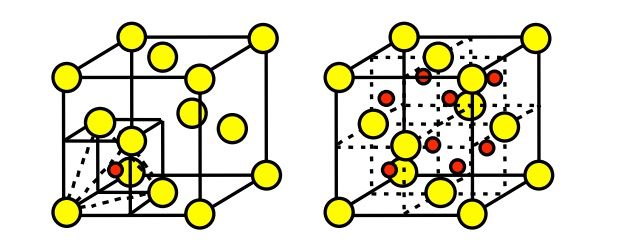
From this diagram of the rock salt structure ( N a C l) we see that both the chloride and sodium ions have the same environment That is to say, they each have the same number of neighbours at every distance, except that the charges are opposite However, I've always been taught to consider the chloride ions as forming a facecentred cubic (fcc) or cubic close packed (ccp) lattice, with sodium ions filling octahedral holes. Rock Salt As its name implies the archetypal rock salt structure is NaCl (table salt) In common with the zinc blende structure, rock salt consists of two interpenetrating facecentered cubic lattices However, the second lattice is offset 1/2a along the unit cell axis. 1217 Iron oxide (FeO) has the rock salt crystal structure and a density of 570 g/cm 3 (a) Determine the unit cell edge length (b) How does this result compare with the edge length as determined from the radii in Table 123, assuming that the Fe 2 and O 2– ions just touch each other along the edges?.
"Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement The symmetry is the same as that of methane The sulfur atom is in the 6 oxidation state while the four oxygen atoms are each in the −2 state. The rock salt structure is slightly favored thermodynamically 11 In the context of band theory, explain why Mg is a metal, despite the fact that it has a filled s 2 electron configuration The valence orbitals for Mg are 3s and 3p When the atom condenses into the solid state all of the valence orbitals overlap to form bands and the bottom of. Halides also tend to have a highly ordered molecular structure and a high degree of symmetry The most wellknown mineral of this group is halite (NaCl) or rock salt Figure 10d6 Halite or rock salt.
Halite is the mineral name for the substance that everyone knows as "salt" Its chemical name is sodium chloride, and a rock composed primarily of halite is known as "rock salt" Salton Sea Halite Halite from the Salton Sea, California Specimen is approximately 4 inches (10 centimeters) across Halite structure This diagram shows the. = n " (AFe AO) VCNA Since the crystal structure is rock salt, n' = 4 formula units per unit cell Using the ionic radii for Fe2 and O2from Table 123, the unit cell volume is computed. A "rock salt" structure is simple cubic, with an anion at each corner of the cube, and the cation in the middle of the cube (and vice versa) Coordination number is the number of nearest neighbors in the lattice There are 6 O at the corners, all the same distance from the caged Ni in the middle Coordination number is therefore 6.
– 66 (Oh) Cl – with Na in all Oh holes Polyhedra – Edgesharing and octahedra All octahedral holes in a cubic close packing are occupied by counterions Both ions show octahedral coordination (CN = 6). This is the same material that makes up the tiny crystals of table salt The cubic arrangement of atoms, and the cubes that are visible in these large Halite crystals and the small grains of table salt, is called the rock salt structure. A "rock salt" structure is simple cubic, with an anion at each corner of the cube, and the cation in the middle of the cube (and vice versa) Coordination number is the number of nearest neighbors in the lattice There are 6 O at the corners, all the same distance from the caged Ni in the middle Coordination number is therefore 6.
The silver iodides were selfassembled into rocksalt structures instead of conventional β or γ phases inside MWCNTs with inner diameters of 4–8 nm, while a helix structure instead of any known structures predicted in the p–T diagram of silver iodides was obtained within SWCNTs with an inner diameter around 14 nm The structure and. In the rocksalt or sodium chloride (halite) structure, each of the two atom types forms a separate facecentered cubic lattice, with the two lattices interpenetrating so as to form a 3D checkerboard pattern Alternately, one could view this structure as a facecentered cubic structure with secondary atoms in its octahedral holes. Rock Salt vs Calcium Chloride It basically comes down to rock salt vs calcium chloride when choosing an ice and snow removal product Rock salt is the more common name for sodium chloride Both products have their advantages and disadvantages.
Rock Salt As its name implies the archetypal rock salt structure is NaCl (table salt) In common with the zinc blende structure, rock salt consists of two interpenetrating facecentered cubic lattices However, the second lattice is offset 1/2a along the unit cell axis. Rock salt structure Quick Reference A type of ionic crystal structure in which the cations have a facecentred cubic arrangement, with anions occupying all the octahedral holes. A new topological crystalline insulator material, SnSe in the rock‐salt structure, is obtained using molecular beam epitaxyThe thermodynamically unstable rock‐salt SnSe phase is stabilized in epitaxial films up to nm by a Bi 2 Se 3 substrate Dirac surface states are observed at both the and the points using angle‐resolved photoemission spectroscopy;.
Describes the rock salt crystal structure, which is the structure of FeO Made by faculty at the University of Colorado Boulder, Department of Chemical & Bio. The Rock Salt structure This the structure adopted by Sodium Chloride, NaCl It is based on the fcc array of the large chloride anions, and the sodium cations occupy all the octahedral holes in the fcc lattice However, it could also be seen as an fcc array of sodium ions, with the anions in all the octahedral holes. A binary solid (AB) has a rock salt structure If the edge length is 500 pm, and radius of a cation is 80 pm Find the radius of an anion.
Compound AB has rock salt type structure The formula weight of AB is 6023 Y amu and closest AB distance is Y 1/3 nm, where Y is any arbitrary number (a) Find the density of the lattice (b) If density of lattice is found out to be Kg / m 3 then predict the type of defect. One finds rock salt deposits ringing dry lake beds, inland marginal seas, and enclosed bays and estuaries in arid regions of the world At various times in the geologic past, very large bodies of water (such as the Mediterranean Sea and an old ocean that sat where the Atlantic Ocean sits now) also evaporated and made enormous deposits of rock salt. Table Salt Large cubic crystals of sodium chloride (NaCl) are often found in nature (Halite);.
Rock salt structure ( NaCl) Rock salt structure ( NaCl) Here anions form FCC and cations occupy all the octahedral void Here coordination number of cation as well as anion is 6 Unit cell formula is Na4Cl4 FigCsCl structure Here anions forms simple cubic lattice and cation is present at body center. The rocksalt structure Structurally, each ion in sodium chloride is surrounded and held in tension by six neighboring ions of opposite charge The resulting crystal lattice is of a type known as simple cubic, meaning that the lattice points are equally spaced in all three dimensions and all cell angles are 90°. Halite (/ ˈ h æ l aɪ t / or / ˈ h eɪ l aɪ t /), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (Na Cl)Halite forms isometric crystals The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic.
Halides also tend to have a highly ordered molecular structure and a high degree of symmetry The most wellknown mineral of this group is halite (NaCl) or rock salt Figure 10d6 Halite or rock salt. 1213 Calculate the density of FeO, given that it has the rock salt crystal structure Solution We are asked to calculate the theoretical density of FeO This density may be computed using Equation (121) as !. Rock salt structure ( NaCl) Rock salt structure ( NaCl) Here anions form FCC and cations occupy all the octahedral void Here coordination number of cation as well as anion is 6 Unit cell formula is Na4Cl4 FigCsCl structure Here anions forms simple cubic lattice and cation is present at body center.
The carrier control resulted in a domeshaped Tc as a function of electron carrier density In addition, the Tc was significantly sensitive to epitaxial strain in spite of the highly symmetric crystal structure This rocksalt superconducting LaO could be a building block to design novel superlattice superconductors. "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement The symmetry is the same as that of methane The sulfur atom is in the 6 oxidation state while the four oxygen atoms are each in the −2 state. The rock salt structure is face centered cubic and for GaN also contains equal amounts gallium and nitrogen Rock salt has the symmetry of Fm3m ( space group 225 ) with N 1 and Ga at Wyckoff positions a and b respectively ( see Appendix A ) The conventional.
The rock salt structure is face centered cubic and for GaN also contains equal amounts gallium and nitrogen Rock salt has the symmetry of Fm3m ( space group 225 ) with N 1 and Ga at Wyckoff positions a and b respectively ( see Appendix A ) The conventional. However, there are quite a few differences beyond that. Zinc oxide represents an important material with a wide variety of applications At the temperature T = K and standard pressure p° = 100 kPa ZnO exists in the hexagonal wurtzite structure, which undergoes a transformation to rock salt polymorph under the pressure of ≈9 GPaA consistent set of thermodynamic functions for ZnO in the highpressure rock salt modification has been.
Cite this chapter as Bilz H, Kress W (1979) Metal Oxides (Rock Salt Structure) In Phonon Dispersion Relations in Insulators Springer Series in SolidState Sciences, vol 10. (a) (100) plane for the rock salt crystal structure (b) (110) plane for the cesium chloride crystal structure (c) (111) plane for the zinc blende crystal structure. – 66 (Oh) Cl – with Na in all Oh holes Polyhedra – Edgesharing and octahedra All octahedral holes in a cubic close packing are occupied by counterions Both ions show octahedral coordination (CN = 6).
A rock salt shotgun round sprays out bits and pieces of salt like pellets from a conventional shot shell This means there is a nonzero chance that a crystal could penetrate a portion of the body that might result in grievous bodily harm or death, such as eye socket The scattering effect of a rock salt shotgun round may be one of its. "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement The symmetry is the same as that of methane The sulfur atom is in the 6 oxidation state while the four oxygen atoms are each in the −2 state. Compute the atomic packing factor for the rock salt crystal structure in which r C/r A = 0414 Solution This problem asks that we compute the atomic packing factor for the rock salt crystal structure when r C/r A = 0414 From Equation 32 APF = VS VC With regard to the sphere volume, V S, there are four cation and four anion spheres per unit.
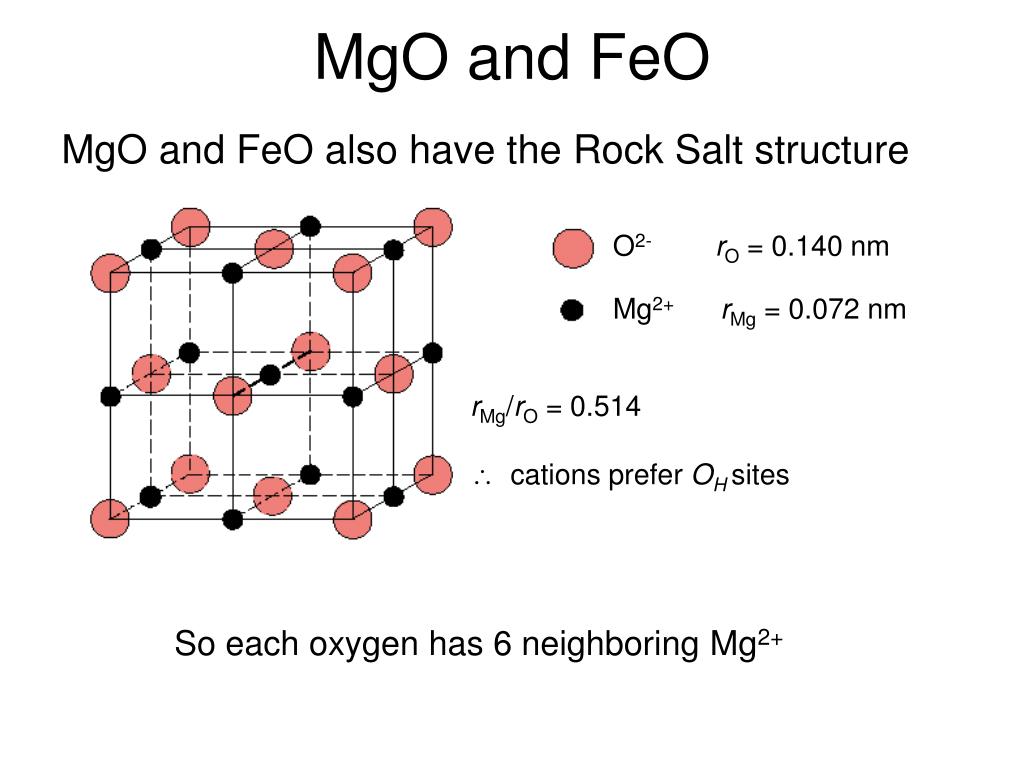
Solution (a) This part of the problem calls for us to determine the unit cell edge length. Compute The Atomic Packing Factor For The Rock Salt Crystal Structure In Which RC/rA=0414 Question Compute The Atomic Packing Factor For The Rock Salt Crystal Structure In Which RC/rA=0414 This problem has been solved!. In the first structure (Figure 2A) the material shown is magnesia (MgO), though the structure itself is referred to as rock salt because common table salt (sodium chloride, NaCl) has the same structure In the rock salt structure each ion is surrounded by six immediate neighbours of the opposite charge (eg, the central Mg 2 cation, which is.
See the answer Compute the atomic packing factor for the rock salt crystal structure in which rC/rA=0414. Solid 'AB' has NaCl structure, otherwise known as Rock salt structure Therefore, same type of atoms (for example 'A' atoms) are arranged in FCC pattern (in which all the 8 corners and all the 6 face centers are occupied) while other type of atoms ('B' atoms) occupy all the 12 edges as well as at the center of the unit cell. This confirms the topological.
The rock salt structure Alkali halides that crystallize with the "rocksalt" structure exemplified by sodium chloride can be regarded either as a FCC structure of one kind of ion in which the octahedral holes are occupied by ions of opposite charge, or as two interpenetrating FCC lattices made up of the two kinds of ions. Rock Salt Structure In the rock salt crystal structure of the molecule, the negative ions of the compound occupy the eight corners of the unit cell and the six centres of the faces and positive. Halite (/ ˈ h æ l aɪ t / or / ˈ h eɪ l aɪ t /), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (Na Cl)Halite forms isometric crystals The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic.
This same basic structure is found in many other compounds and is commonly known as the halite or rocksalt crystal structure It can be represented as a facecentered cubic (fcc) lattice with a twoatom basis or as two interpenetrating face centered cubic lattices The first atom is located at each lattice point, and the second atom is located. The carrier control resulted in a domeshaped Tc as a function of electron carrier density In addition, the Tc was significantly sensitive to epitaxial strain in spite of the highly symmetric crystal structure This rocksalt superconducting LaO could be a building block to design novel superlattice superconductors. Salt, also known as sodium chloride, has many end uses Virtually every person in the world has some direct or indirect contact with salt daily People routinely add salt to their food as a flavor enhancer or apply rock salt to walkways to remove ice in the winter.
The rocksalt structure is a simple crystal structure of the form AX, it is adopted by sodium chloride In the case of NaCl, it is a fcc array of anions with the cations inside the octahedral holes It can also be viewed as fcc array of cations with an anion in each octahedral hole, they both produce the same pattern. To summarize, the rock salt structure has ccp/fcc anions with octahedral sites fully occupied by cations and tetrahedral sites empty Each cation is surrounded by six anions and. Rock salt and table salt are both salts in that they both consist mostly of sodium chloride Because they are composed of the same chemical, rock salt and table salt provide the same flavor;.
Safe Step Rock Salt Ice Melter Sodium Chloride (Rock Salt) Melts Ice Down To 5 F / 15 C 50 Lbs 47 out of 5 stars 17 $3999 $ 39 99 FREE Shipping Only 17 left in stock order soon More Buying Choices $2674 (7 new offers). Solid 'AB' has NaCl structure, otherwise known as Rock salt structure Therefore, same type of atoms (for example 'A' atoms) are arranged in FCC pattern (in which all the 8 corners and all the 6 face centers are occupied) while other type of atoms ('B' atoms) occupy all the 12 edges as well as at the center of the unit cell. Sodium Chloride (Rock salt) Type Structure The sodium chloride structure is composed of Na and Cl ions The number of sodium ions is equal to that of Cl ions The radii of Na and Cl ions 95 pm and 181 pm giving the radius ratio of 0524 The radius ratio of 0524 for NaCl suggest an octahedral void.
Table Salt Large cubic crystals of sodium chloride (NaCl) are often found in nature (Halite);. The rock salt structure Alkali halides that crystallize with the "rocksalt" structure exemplified by sodium chloride can be regarded either as a FCC structure of one kind of ion in which the octahedral holes are occupied by ions of opposite charge, or as two interpenetrating FCC lattices made up of the two kinds of ions. Zinc oxide represents an important material with a wide variety of applications At the temperature T = K and standard pressure p° = 100 kPa ZnO exists in the hexagonal wurtzite structure, which undergoes a transformation to rock salt polymorph under the pressure of ≈9 GPaA consistent set of thermodynamic functions for ZnO in the highpressure rock salt modification has been.
Rock salt structure • Sodium chloride (NaCl) is the most common • Rc/Ra = • CN=6 for both cations and anions • Unit cell FCC arrangement of anions with one cation at center of each of 12 cube edges • Two interpenetrating FCC lattices A unit cell of rock salt AXtype crystal structure (continue) Cesium chloride structure. Halite, commonly known as rock salt, is a type of salt, the mineral form of sodium chloride Halite forms isometric crystals The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates The name halite is deri.
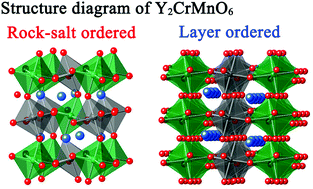
Two Types Of B Site Ordered Structures Of The Double Perovskite Y2crmno6 Experimental Identification And First Principles Study Inorganic Chemistry Frontiers Rsc Publishing
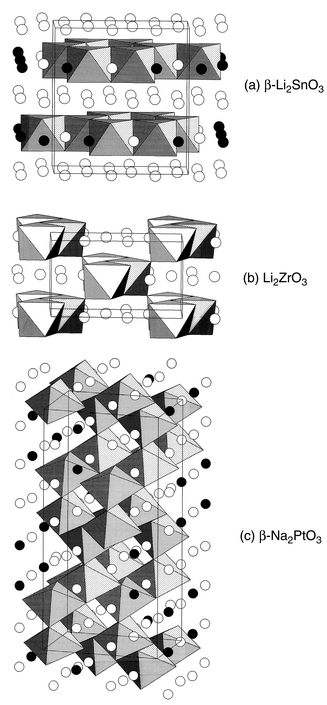
A Review Of Cation Ordered Rock Salt Superstructure Oxides Journal Of Materials Chemistry Rsc Publishing Doi 10 1039 Bf

A Schematic Of The Lattice Structure Of Rock Salt Snse B Layered Download Scientific Diagram
Rock Salt Structure のギャラリー

Rock Salt Structure Youtube

Novel Route From A Wurtzite To A Rock Salt Structure In Coo Nanocrystals In Situ Transmission Electron Microscopy Study The Journal Of Physical Chemistry C X Mol
Pubs Acs Org Doi Pdf 10 1021 Acs Chemmater 5b
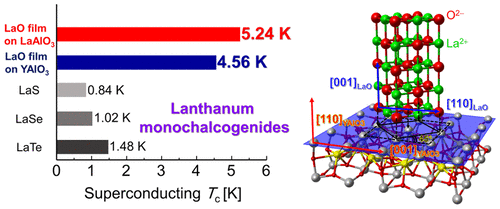
Superconductivity Of Rock Salt Structure Lao Epitaxial Thin Film Journal Of The American Chemical Society X Mol
Welcome To Chem Zipper Com Sodium Chloride Rock Salt Type Structure

Energetics Of Point Defects In Rocksalt Structure Transition Metal Nitrides Thermodynamic Reasons For Deviations From Stoichiometry Sciencedirect

Structure Of Ionic Compounds Study Material For Iit Jee Askiitians

14 In Rock Salt Type Structure Cations Radius R Occupy Octahedral Holes In The Fcc Of

7 1 Crystal Structure Chemistry Libretexts
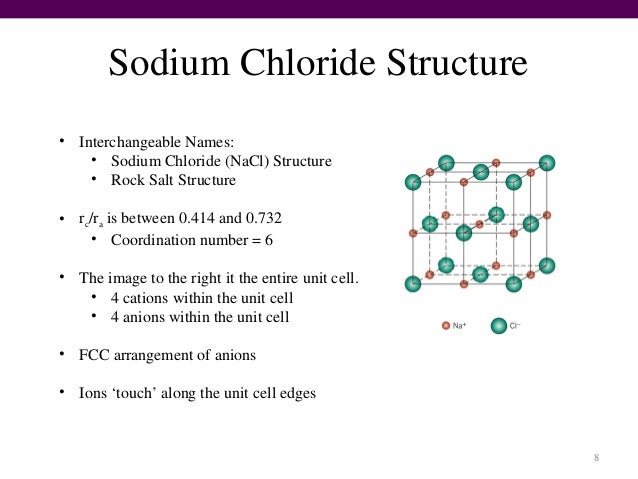
Module 4
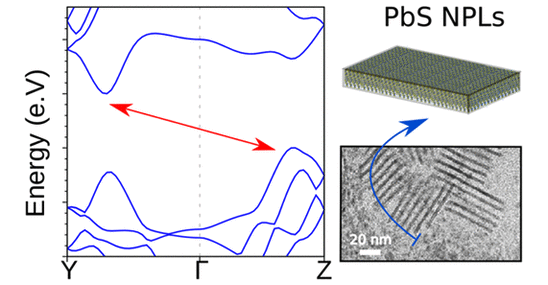
Ivan Mora Sero Morphology And Band Structure Of Orthorhombic Pbs Nanoplatelets An Indirect Band Gap Material Experimental Theoretical Study Of How Pbs Npts Departs From Rock Salt Structure And Their Implications Cienciauji Openaccess
Transition Metal Oxides Rock Salt And Rutile Metal Metal Bonding Ppt Video Online Download
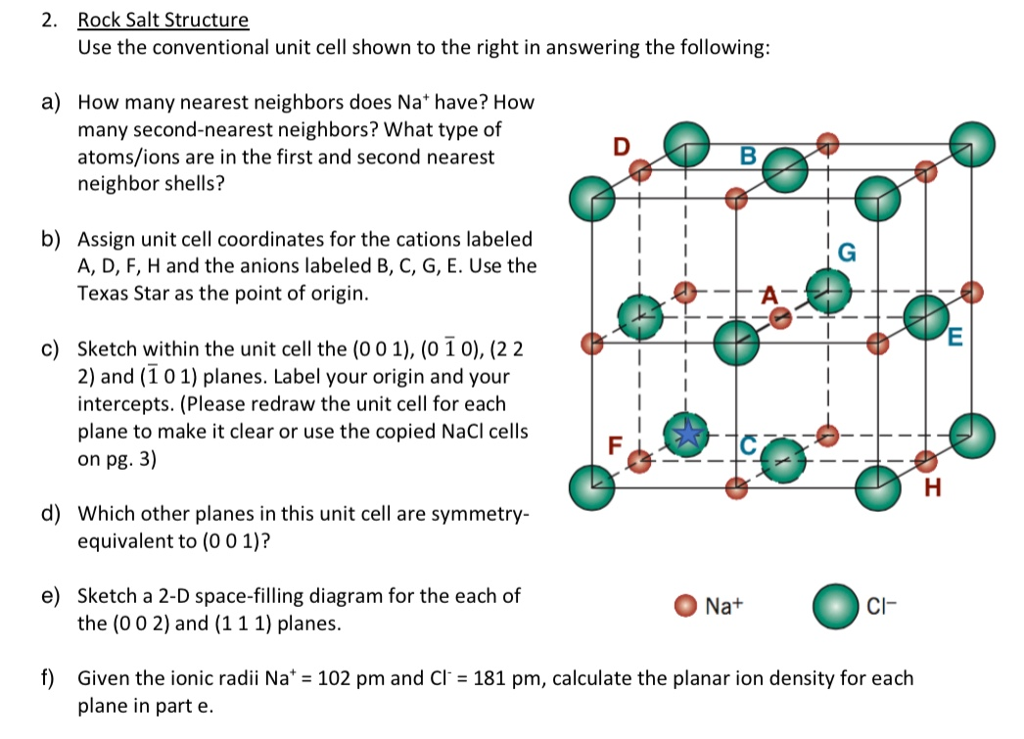
Solved 2 Rock Salt Structure Use The Conventional Unit C Chegg Com

In A Solid Having Rock Salt Structure If All Atoms Touching One Body Diagonal Plane Are Removed Except At Body Chemistry The Solid State Meritnation Com

Engr 145 Exam 2 Flashcards Quizlet

Rock Salt Nacl B1 Structure Ab Cf8 225 A B

Ceramic Composition And Properties Britannica

Figure 3 From Prediction Of Rock Salt Structure Of Inn 32 Nanoparticles From First Principles Calculations Semantic Scholar
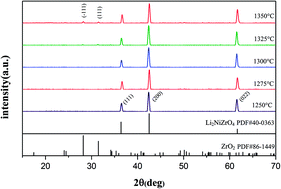
A Novel Microwave Dielectric Ceramic Li2nizro4 With Rock Salt Structure Rsc Advances Rsc Publishing
Q Tbn And9gctkuz2ujbxxno0iyoooio0jun6vucy4u Guy0nidzgadjjxwsea Usqp Cau

Materials Science Tutorials Ceramics

Sodium Chloride Rock Salt Halite Table Salt Chemical Structure Stock Vector Illustration Of Table Chloride
Chapter 12 Structures Properties Of Ceramics Ppt Download

Disordered Rock Salt And Transition Metal Anodes Engineering The Batteries Of The Future
Www Uio No Studier Emner Matnat Kjemi Nedlagte Emner Mef3000 H06 Undervisningsmateriale West 1 17 Pdf
Crystal Systems
Metastable And Nanosize Cation Disordered Rocksalt Type Oxides Revisit Of Stoichiometric Limno2 And Namno2 Journal Of Materials Chemistry A Rsc Publishing

Rock Salt Nacl B1 Structure Ab Cf8 225 A B
15 Rock Salt Ceramic Crystal Structure Youtube

The Structure That Started It All Rock Salt Crystallography365
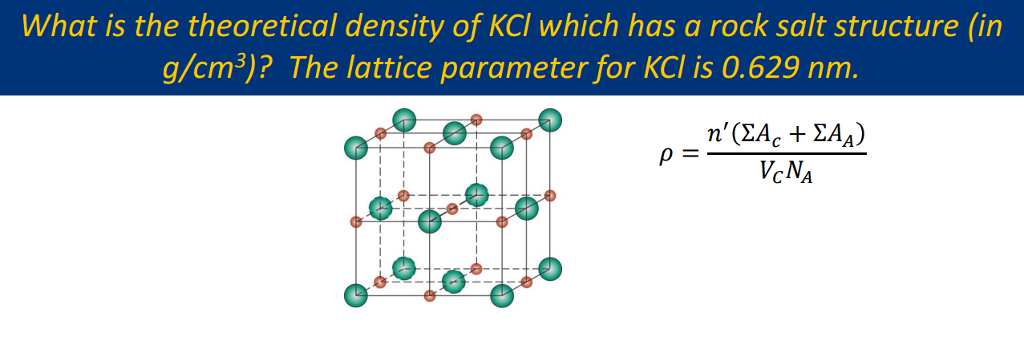
Solved What Is The Theoretical Density Of Kcl Which Has A Chegg Com
Solids Practice Problems Answers
In A Solid Ab Having Nacl Structure A Atoms Occupy Solved
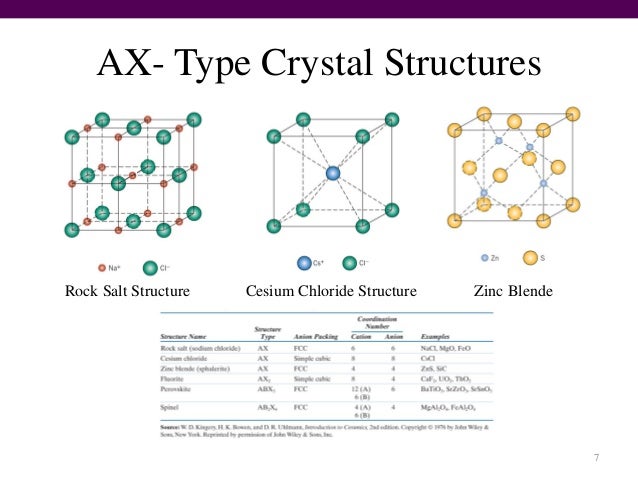
Module 4

Phase Transition In Sic From Zinc Blende To Rock Salt Structure And Implications For Carbon Rich Extrasolar Planets In American Mineralogist Volume 102 Issue 11 17
Tikalon Blog By Dev Gualtieri
Crystal Structures Of A Rock Salt Type And B Zinc Blend Type Download Scientific Diagram
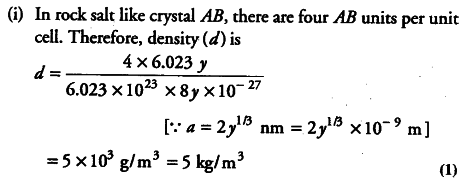
The Crystal Ab Rock Salt Structure Has Molecular Weight 6 023 Cbse Class 12 Chemistry Learn Cbse Forum

New Rock Salt Related Oxides Li3m2ruo6 M Co Ni Synthesis Structure Magnetism And Electrochemistry Sciencedirect
Q Tbn And9gctdrhwoklvjgqqfhxb6ag3c2ilfuxowowfithu X2mwsdmdgxpd Usqp Cau

Objectives Template
Http Phome Postech Ac Kr User Aemn Download Lec 03 Pdf
Ph0kqynfea3 Hm
Chemical Salt Structure Stock Illustrations 477 Chemical Salt Structure Stock Illustrations Vectors Clipart Dreamstime
Www Uio No Studier Emner Matnat Kjemi Nedlagte Emner Mef3000 H06 Undervisningsmateriale West 1 17 Pdf

Objectives Template

In The Rock Salt Structure The Number Of Formula Units Per Unit Cell Is Equal To Youtube

Sodium Chloride Rock Salt Halite Table Stockvector Rechtenvrij

Crystal Structure Of Rock Salt Nacl Ions Of The Same Charge Are Download Scientific Diagram

Relative Stability Electronic Structure And Magnetism Of Mnse In Rocksalt And Zinc Blende Structures Sciencedirect

Charged Rock Salt Structure Where Two Elements Of The Neutral Rock Salt Download Scientific Diagram

Structure Types Condensed Matter Physics Rudi Winter S Web Space

60 English How Many Effective Na And Cl Jons Are Present Respectively In A Unit Cell

Structures Of Simple Ionic Compounds Every Science
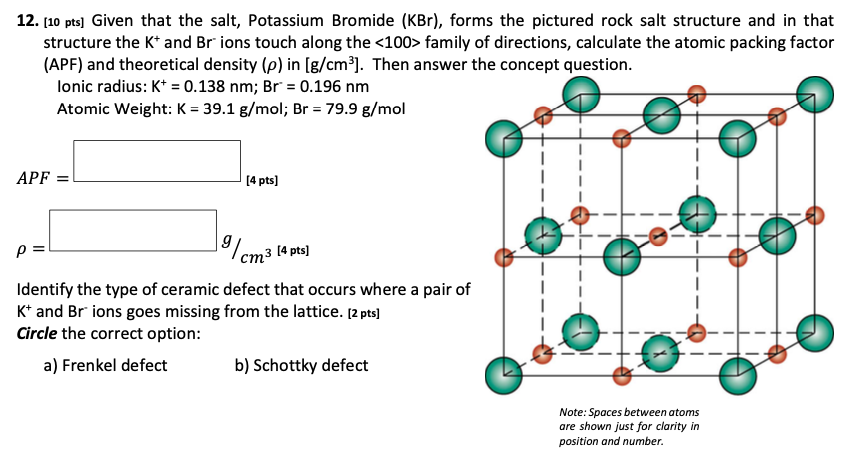
Solved 12 10 Pts Given That The Salt Potassium Bromid Chegg Com
Salt Deposits
Lithiation Driven Structural Transition Of Vo2f Into Disordered Rock Salt Lixvo2f Rsc Advances Rsc Publishing

On The Influence Of Tetrahedral Covalent Hybridization On Electronic Band Structure Of Topological Insulators From First Principles Journal Of Applied Physics Vol 117 No 4

Chemistry
6 11a Structure Rock Salt Nacl Chemistry Libretexts

A Binary Solid A B Has A Rock Salt Structure If The Edge Length Is 500 Picometre And Radius Of Chemistry The Solid State Meritnation Com

Nacl Mgo Lif Mns And Feo Cesium Chloride Structure Cscl The Coordination Number Course Hero

Consider The Rock Salt Structure In The Fi Clutch Prep
Search Q Rock Salt Structure Coordination Number Tbm Isch

Chemistry

Q In A Solid Ab Having Rock Salt Structure If All The Atoms Touching 1 Body

An Ionic Solid A B Crystallizes In Rock Salt Type Structu
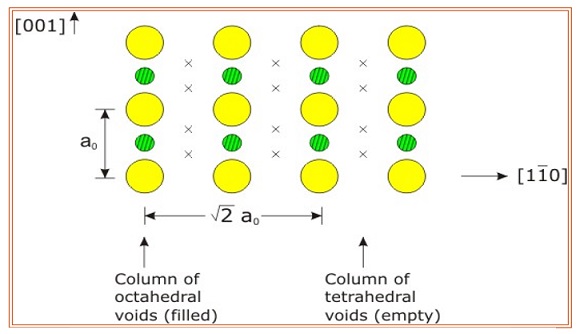
Learn Examples On Rock Salt Structure Meaning Concepts Formulas Through Study Material Notes Embibe Com

Sodium Chloride Rock Salt Halite Table Salt Crystal Structure Stock Photo Picture And Royalty Free Image Image

Rock Salt Structure Chloride Lattice Or Sodium Lattice Chemistry Stack Exchange
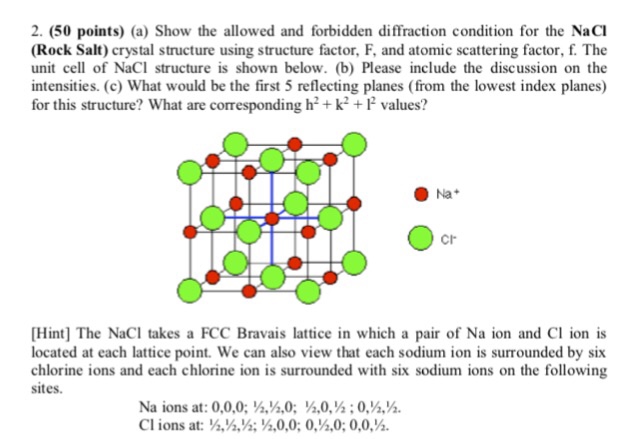
Solved Show The Allowed And Forbidden Diffraction Conditi Chegg Com

Color Online A 110 Plane Of The Rock Salt Structure Of Nio And Mno Download Scientific Diagram
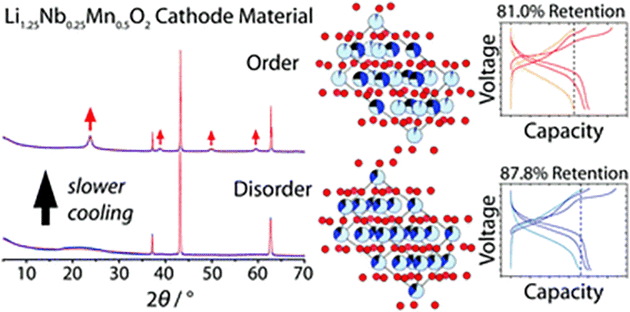
Short Range Ordering In A Battery Electrode The Cation Disordered Rocksalt Li1 25nb0 25mn0 5o2 Chemical Communications Rsc Publishing
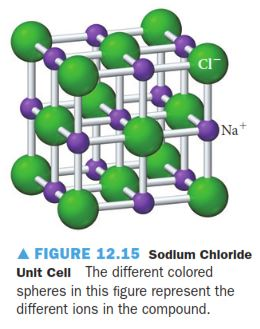
Solved Consider The Rock Salt Structure In Figure 12 15 Chegg Com
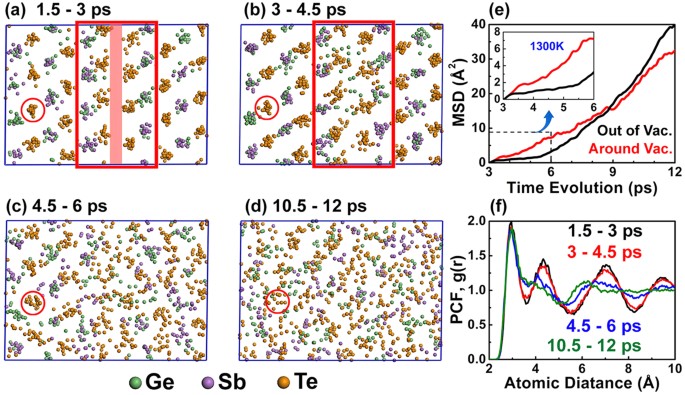
Vacancy Structures And Melting Behavior In Rock Salt Gesbte Scientific Reports

Figure 1 From Prediction Of Rock Salt Structure Of Inn 32 Nanoparticles From First Principles Calculations Semantic Scholar

Physical Chemistry Solid State Assertion In Rock Salt Structure The Sodium Ions Occupy Octahedral Voids Reason The Radius R Kunduz

Molecular Structure Rock Salt Stock Photo Download Image Now Istock

Solid State Chemistry
Www Wiley Vch De Books Sample C01 Pdf
Http Www Rpi Edu Galld Publications Pdf Files Gall 134 Pdf
Explain How The Tetrahedral Voids Formed By Ccp Arrangement Of Cl Ions In Rock Salt Structure Are Vacant Yys96ctt Chemistry Topperlearning Com

The Common Rocksalt Crystal Structure Of The Rens The Large Spheres Download Scientific Diagram
Crystal Structure And Crystallography Of Materials Ppt Download
Atoms In Motion Atoms In Motion Chapter 4 Salts
Q Tbn And9gcr46y3lrbq2gftw35aivq7vbcstldwg2xff4ytk9zvxiexh9ssq Usqp Cau

Chemistry Rock Salt Structure

Number Of Atoms In Nacl Unit Cell Chemistry Stack Exchange
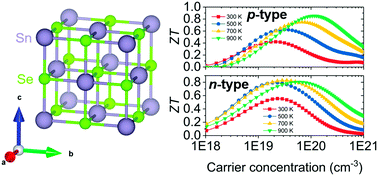
Thermoelectric Transport Properties Of Rock Salt Snse First Principles Investigation Journal Of Materials Chemistry C X Mol
Http Pubs Acs Org Doi Pdf 10 1021 Acsomega 7b

A Schematic Of The Lattice Structure Of Rock Salt Snse B Layered Download Scientific Diagram
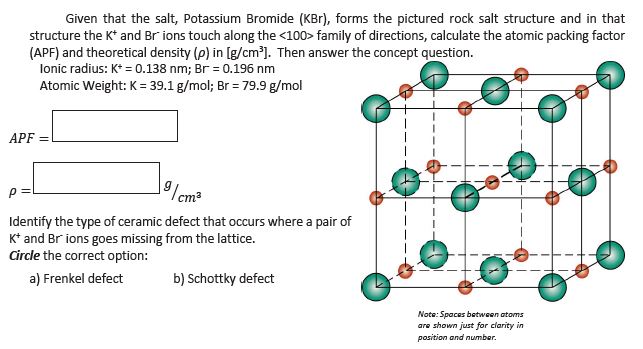
Answered Given That The Salt Potassium Bromide Bartleby

A Binary Solid Abab Has A Rock Salt Structure If The Edge Length Is Pm And Radius Of The Brainly In

In An Idaeal Closet Rock Salt Structure Edge Length A Which Of The Following Expression Youtube

Consider The Rock Salt Structure In The Fi Clutch Prep

High Capacity Electrode Materials For Rechargeable Lithium Batteries Li3nbo4 Based System With Cation Disordered Rocksalt Structure Pnas
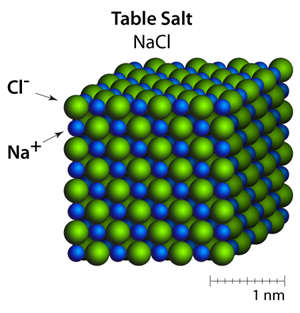
Atoms In Motion Atoms In Motion Chapter 4 Salts

1 A Distorted Rocksalt Structure Of Metstable Gete B Metastable Download Scientific Diagram
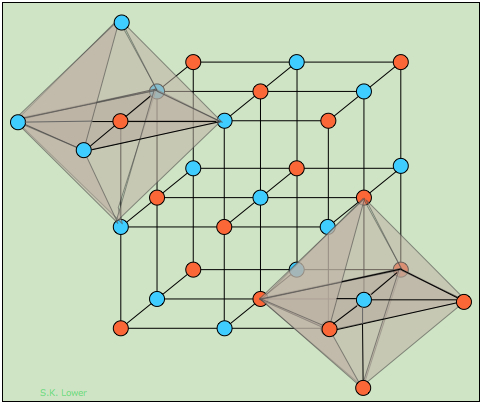
The Rock Salt Structure

Unveiling Composition Crystal Structure Dependent Electrochemical Behaviors Via Experiments And First Principles Calculations Rock Salt Nicoo2 Vs Spinel Ni1 5co1 5o4 Sciencedirect
Ppt Ceramics Powerpoint Presentation Free Download Id



